Advances in Animal and Veterinary Sciences
Research Article
The Pelvic Girdle of Cow: Ultrasonographic Description
Ahmed Sharshar1*, Shaaban Gadallah1, Mohamed Elsunsafty1, Mustafa Fadel2
1Department of Surgery, Anesthesiology, and Radiology. Faculty of Veterinary Medicine, University of Sadat City, Egypt; 2Department of Diagnostic Imaging and Endoscopy, Animal Reproduction and Research Institute, Egypt.
Abstract | This study was carried out to offer a detailed ultrasonographic description of the pelvic girdle mature cows. Twenty mature and clinically healthy cows of the local (Balady) breed were examined ultrasonographically. A complete pelvic examination was carried out after animal restraint. All components of the pelvic girdle including osseous structures, joints, ligaments and tendons were examined and evaluated both externally and internally. For each measured item, the obtained results were compared between the right and left sides. The pelvic tuberosities (sacral tuber, coxal tuber, and ischial tuber) appeared as convex arches with a variant degree of irregularities on the surfaces. The bony outside surfaces of iliac wing and body and the ischium appeared as thin hyperechoic concave arches; while the inside surfaces of the tabula of the Ischium, pubis, medial aspect of the acetabulum and the iliac body appeared as thin hyperechoic convex arches. Pelvic articulations including hip joint and sacroiliac joint appeared as anechoic band bounded by hyperechoic arches. The pelvic tendon and ligaments appeared as echogenic structures with a longitudinal linear fiber pattern. The obtained results can be used as a guide for pelvic examination in mature cows.
Keywords | Cow, Pelvis, Transcutaneous, Transrectal, Ultrasonography
Received | September 06, 2019; Accepted | January 12, 2020; Published | February 20, 2020
*Correspondence | Ahmed Sharshar, Department of Animal Surgery, Anesthesiology, and Radiology, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Egypt; Email: [email protected]
Citation | Sharshar A, Gadallah S, Elsunsafty M, Fadel M (2020). The pelvic girdle of cow: Ultrasonographic description. Adv. Anim. Vet. Sci. 8(2): 190-197.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.2.190.197
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Sharshar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Pelvic injuries occur commonly in cattle (Weaver, 1969; Larcombe and Malmo, 1989; Baird and Baird, 1995; Starke et al., 2007). Some of these injuries in terms of bone fractures may lead to disruption of the normal pelvic regularity and dimensions with subsequent reduction of the animal’s reproductive capacity (Divers and Peek, 2008) and consequential increase of animal culling rates (Weaver, 1969; Larcombe and Malmo, 1989; Gundelach et al., 2013). The pelvis is structurally complex, and this makes the diagnosis of its injuries a great challenge in large animal practice (Whitcomb, 2012; Head, 2014).
Radiographic examination is the only feasible means to confirm a specific pelvic injury of the calves after primary clinical examination (Weaver, 1969; Nelson and Kneller, 1985). From the technical point of view, it may subject the animal to the risk of luxation and fracture displacement, which can occur from the specific positioning required for image production (Farrow, 1999; Ferguson, 1997; Starke et al., 2007). Moreover, radiographic equipment is not available to many veterinarians in bovine practice (Kofler, 2009).
Ultrasonography has been developed and become an established diagnostic tool for examination of the bovine musculoskeletal system during the past 20 years (Tryon and Clark, 1999; Grubelnik et al., 2002; Kofler, 1995, 1996, 1999, 2000; Saule et al., 2005; Starke et al., 2007; Taguchi et al., 2011). It has important advantages including its availability, safety and convenience in the field (Sharshar et al., 2018). Besides, it can be used to distinguish some pelvic affection (Greenough et al., 1981; Larcombe and Malmo, 1989; Baird and Baird, 1995; Starke et al., 2007). The knowledge of the normal ultrasonographic anatomy of the cow pelvic girdle is very essential for the evaluation and diagnosis of its various affections. To our knowledge, information on the normal ultrasonographic anatomy of cattle pelvis is very scarce and incomplete (Grubelnik et al., 2002), considering the distribution and economic importance of cattle throughout the world. The present study aims to record the normal sonographic anatomy of the cow’s pelvic girdle, to establish a reference parameter.
Materials And Methods
The study protocol followed the faculty guidelines All animal-handling procedures were according to the regulation of Institutional Animal Care and Use Committee (IACUC), Faculty of Veterinary Medicine, University of Sadat City, Egypt (Approval number: VUSC-009-1-19). This study was carried out on 20 mature and clinically healthy cow of local breed (Balady breed) with no previous history of hindlimb lameness or pelvic affection. The mean age was 6.5 years (range, 5 to 8 years) and mean body weight was 375 kg (range, 300 to 450 kg). All animals were quietly inspected in the stall and their back posture was evaluated while they were standing and walking. All animals included in the study were free from any abnormality or lameness (score 1 according to the scoring system mentioned by Sprecher et al., 1997).
The examined animals were controlled in a stanchion and chemically restrained by intramuscular administration of 2% xylazine hydrochloride (0.05 mg/kg B.W. I/M.). Analgesia was achieved by induction of caudal posterior epidural analgesia using 2% lidocaine hydrochloride (1 ml/100 kg B.W). The hair overlaying each hemipelvis (the area bounded cranially by sacral tuber and tuber coxae, laterally by greater trochanter, medially by the sacrum and caudally by ischial tuber) was clipped followed by cleansing the skin using ethyl alcohol and spreading the ultrasonographic gel. Evacuation of feces from the rectum was performed directly before the transrectal examination.
The examination was carried out using ultrasonographic unit (Esaote MyLab™One 507 made in Italy) equipped with 6.6 to 18 MHz linear array tendon, rectal linear and micro-convex probes. Both transcutaneous and transrectal examinations were performed for a complete evaluation of the pelvis. The examination was performed according to the technique that was previously described in equine by Walker et al. (2012), Head (2014), Whitcomb and Vaughan (2015). During transcutaneous examination, the following structures were examined and evaluated: (1) sacral tubers (ST) including its shape, depth from the skin surface, the distance between both tubers and the distance from the medial aspect of each of them to the most dorsal point of the first sacral spinous process; (2) dorsal part of the dorsal sacroiliac ligament-thoracolumbar fascia combination (D-DSIL-TLF) including its shape, cross-sectional area, and thickness; (3) the lateral portion of the dorsal sacroiliac ligament (L-DSIL) including its shape, thickness and its attachment to the lateral sacral crest; (4) iliac wing (IW); (5) tuber coxae (TC); (6) lateral aspect of the iliac body (IB); (7) hip joint (HJ); (8) greater trochanter (GT); (9) ischium (IS) and (10). ischial tuber (IT).
During transrectal examination, the following structures were examined and evaluated; (1) pelvic symphysis (PS); (2) tabula of the Ischium (TI); (3) obturator foramen (OF) and its contents (blood vessel and nerve); (4) medial aspect of the acetabulum (AC); (5) medial aspect of iliac body (IB); (6) ventral aspect of the sacrum (VS); and (7) the sacroiliac joints (SIJ) including its shape and width. To obtain the best ultrasonographic image, we have followed the rules explained in our previous work concerning the selection of the proper probe type, appropriate frequency and depth for the examination of each structure (Sharshar et al., 2018).
Statistical analyses
For each measured item, the mean, standard error (SE) and range of values were calculated. The differences in values between the right and left sides of the examined animals were analyzed using paired t-test. For all tests, significance was set at P<0.05.
RESULTS
Both sacral tubers appeared in the transverse scan as a smooth convex hyperechoic arch extended lateromedially. It was covered by hypoechoic curvilinear structure extended medially representing the dorsal part of the dorsal sacroiliac ligament-thoracolumbar fascia combination (Figure 1A). The dorsal spinous process of the first sacral vertebrae appeared as a hyperechogenic small rounded area with acoustic shadowing located midway between sacral tubers (Figure 1A). In the longitudinal scan, each sacral tuber appeared as an irregular, slightly convex hyperechoic arch directed craniocaudally with cranial and caudal processes (Figure 1B). The mean distance between both sacral tubers, the mean distance between the medial aspect of both sacral tubers and the most dorsal point of the first sacral spinous process and the mean depth of them from the skin surface are listed in Table 1.
During transverse scan, the dorsal aspect of the dorsal sacroiliac ligament-thoracolumbar fascia combination appeared as a curvilinear hypoechoic to echogenic structure extended lateromedially. Its lateral branch representing the dorsal aspect of the dorsal sacroiliac ligament appeared as a convex hypoechoic arch extended laterally to cover the proximal aspect of the sacral tuber. But, its medial branch representing the thoracolumbar fascia appeared slightly more echogenic V-shaped structure extended laterally to fuse with the medial aspect of the dorsal aspect of the sacroiliac ligament (in all examined animals), and extended medially to connect with the opposite side at the proximal
Table 1: Different measurements of the pelvis in adult cow recorded in this study (mean ± SE).
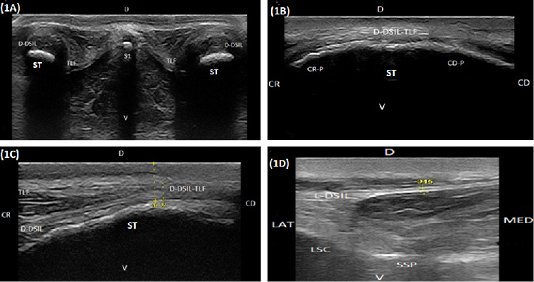
Figure 1: (A) Compound transverse sonogram (10 MHz linear tendon probe, 6 cm depth) at the dorsal aspect of the sacroiliac region of a 6-year-old cow showing sacral tuber (ST), the thoracolumbar fascia (TLF), the dorsal portion of the dorsal sacroiliac ligament (D-DSIL). first sacral spinous process (S1), dorsal direction (D) and ventral direction. (B) Compound longitudinal sonogram (14 MHz linear tendon probe, 5cm depth) at the dorsal area of sacral tuber of a 7- year- old cow showing sacral tuber (ST), the dorsal portion of the dorsal sacroiliac ligament- thoracolumbar fascia combination (D-DSIL-TLF), cranial margin of sacral tuber (CR-P), caudal margin of sacral tuber (CD.P), cranial direction (CR), caudal direction (CD), dorsal direction (D) and ventral direction (V). (C) Compound longitudinal sonogram at the dorsal area of sacral tuber of the same animal in Figure. 1B. showing the dorsal aspect of the dorsal sacroiliac ligament (D-DSIL), thoracolumbar fascia (TLF), sacral tuber (ST), cranial direction (CR), caudal direction (CD), dorsal direction (D) and ventral direction (V). (D) Transverse sonogram (10 MHz linear tendon probe, 6 cm depth) of an 8-year-old cow showing the lateral aspect of the dorsal sacroiliac ligament (L-DSIL), the lateral sacral crest (LSC), the sacral spinous process (SSP), lateral direction (LAT), medial direction (MED), dorsal direction (D) and ventral direction (V).
aspect of the dorsal spinous process of the sacral vertebra (Figure 1A). Cranial to the cranial process of the sacral tuber, the dorsal aspect of the dorsal sacroiliac ligament and the thoracolumbar fascia appeared in the longitudinal scan as double homogenous slightly echogenic layers separating from each other by anechoic zone (Figure 1C). They completely fused at the most dorsal point of the cranial margin of the sacral tuber and proceeded caudally as a single structure (Figure 1B and 1C). The lateral aspect of the dorsal sacroiliac ligament appeared as a regular slightly hypoechoic linear structure with hyperechoic limiting borders. It extended lateroventrally to the vertical axis and inserting at the lateral sacral crest which, appeared as a smooth hyperechoic arch (Figure 1D). The mean thickness and cross-sectional area of the dorsal aspect of the dorsal sacroiliac ligament-thoracolumbar fascia combination at the point of their fusion and the mean thickness of the lateral aspect of the dorsal sacroiliac ligament are listed in Table 1.
The iliac wing appeared as a regular hyperechoic slightly concave arch extending from the sacral tuber medially to the tuber coxae laterally (Figure 2A). The tuber coxae appeared as an irregular hyperechoic linear structure with marked irregularity at its middle portion resembling splintered fractures (Figure 2B). The iliac body was imaged as a slightly concave hyperechoic linear structure extending from tuber coxae cranially and directed caudoventrally to the acetabulum (Figure 2C). The hip joint was localized at the caudoventral end of the iliac body where its surface diverged to be convex. It appeared as an anechoic narrow band bounded dorsally and ventrally by two hyperechoic arches representing the acetabulum and the femoral head (Figure 2C and 2D). Imaging the caudal portion of the hip joint was obscured by the greater trochanter which appeared as a hyperechoic arch (Figure 2D). The ischium appeared as a hyperechoic slightly concave arch extended caudally from the acetabulum to the ischial tuber (Figure 3A). The ischial tuber appeared during both longitudinal and transverse ultrasonographic scans as a hyperechoic irregular slightly convex linear structure covered by slightly an echogenic muscular mass (Figure 3B).
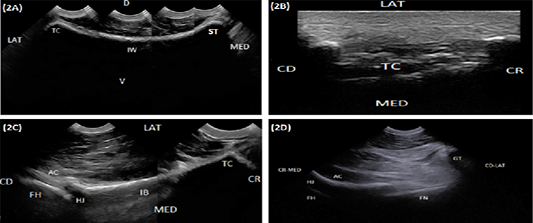
Figure 2: (A) Compound longitudinal sonogram (6.6 MHz micro convex probe, 10 cm depth) in a 5-year-old cow showing iliac wing (IW), sacral tuber (ST), tuber coxae (TC), lateral direction (LAT), medial direction (MED), dorsal direction (D) and ventral direction (V). (B) Longitudinal sonogram (10 MHz linear tendon probe, 5cm depth) in an 8-year-old cow showing tuber coxae (TC) lateral direction (LAT), medial direction (MED), cranial direction (CR) and caudal direction (CD). (C) Compound longitudinal sonogram (6.6 MHz micro convex probe, 10 cm depth) in a 5-year-old cow showing the iliac body (IB), hip joint (HJ), tuber coxae (TC), acetabulum (AC), femoral head (FH), lateral direction (LAT), medial direction (MED), cranial direction (CR) and caudal direction (CD). (D) Oblique sonogram (6.6 MHz micro convex probe, 15 cm depth) in an 8-year-old cow showing the hip joint (HJ), the acetabulum (AC), femoral head (FH), femoral neck (FN), greater trochanter (GT), craniomedial direction (CR-MED), and caudolateral direction (CD-LAT).
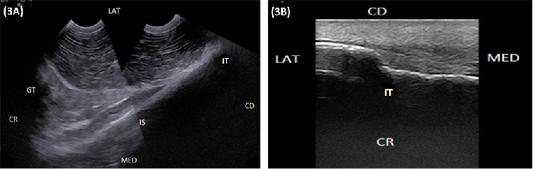
Figure 3: (A) Compound longitudinal sonogram (6.6 MHz micro convex probe, 11 cm depth) in a 6-year-old cow showing the ischium (IS), ischial tuber (IT), greater trochanter (GT). CR, Lateral direction (LAT), medial direction (MED), cranial direction (CR) and caudal direction (CD). (B) longitudinal sonogram (10 MHz linear tendon probe, 5 cm depth) in an 8-year-old cow showing ischial tuber (TI), lateral direction (LAT), medial direction (MED), cranial direction (CR) and caudal direction (CD).
The ischiatic table appeared during longitudinal ultrasonographic scanning as a smooth hyperechoic convex arch extended from the ischial tuber caudally till the ischiatic rim of the obturator foramen cranially (Figure 4A). While in transverse scanning both Tabula of the ischium appeared as hyperechoic lines separated by slightly hypoechoic area represented the pelvic symphysis. The latter was visible at the caudal portion of the pelvic floor and completely disappeared at its cranial half (Figure 4B and 4C). The obturator foramen appeared as an area of mixed echogenicity containing nerve and blood vessels. The obturator nerve appeared as a slightly echogenic structure bounded dorsally by the obturator blood vessel (Figure 4D). The pubis and the pubic rim appeared as a smooth hyperechoic convex arch extending cranially from the pubic margin of the obturator foramen (Figure 4D and 5A). The medial aspect of the acetabulum appeared as a hyperechoic regular and slightly convex line extended craniodorsally to connect with the iliac body. The latter appeared as a hyperechoic smooth linear structure extended craniodorsally from the acetabulum till the sacroiliac joint (Figure 5B). The sacroiliac joint appeared as a narrow hypoechoic to echogenic band bounded dorsolateral and ventromedially by two smooth hyperechoic arches constitutes the iliac wing and the first sacral transverse process respectively. The iliac wing appeared as a smooth hyperechoic margin connected laterally to a concave hyperechoic arch representing the iliac body. A prominent bony lip was imaged at the point of junction between the iliac wing and body. The transverse process of the first sacral vertebrae appeared as a hyperechoic straight to slightly convex arch (Figure 5B and 5B). The mean width of the sacroiliac joint was listed in Table 1. The ventral aspect of the sacrum appeared at its central portion as a straight to slightly convex hyperechoic smooth line (Figure 5D proximal), while its lateral portion contained small areas of mixed echogenicity constituting the ventral sacral foramina (Figure 5D distal).
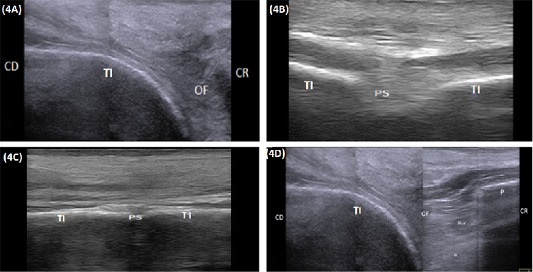
Figure 4: (A) Compound longitudinal sonogram (10 MHz linear rectal probe, 5cm depth) in a 7-year-old cow showing tabula of the ischium (TI), obturator foramen (OF), cranial direction (CR) and caudal direction (CD). (B) Transverse sonogram (10 MHz linear rectal probe, 5cm depth) of the caudal half of the pelvic floor in of the same animal in Figure: 4A showing the pelvic symphysis (PS) and tabula of the ischium (TI). (C) Transverse sonogram (10 MHz linear rectal probe, 5cm depth) of the cranial half of the pelvic floor of the same animal in Figure: 4A showing the pelvic symphysis (PS) and tabula of the ischium (TI). (D) Compound longitudinal sonogram (10 MHz linear rectal probe, 10cm depth) of the of the same animal in Figure 4A showing obturator foramen(OF), blood vessels (BL. V), nerve (N), tabula of the ischium (TI), pubis (P), caudal direction (CD) and cranial direction (CR).
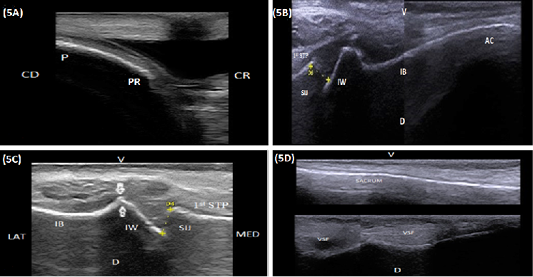
Figure 5: (A) . Longitudinal sonogram (10 MHz linear rectal probe, 5 cm depth) of the pubis (P) in a 7-year-old cow showing the pubic rim (PR), caudal direction (CD) and cranial direction (CR). (B) Longitudinal sonogram (10 MHz linear rectal probe, 5 cm depth) in a 6-year-old cow showing the medial aspect of the acetabulum (AC), the medial aspect of the iliac body (IB), iliac wing (IW), sacroiliac joint (SIJ), first sacral transverse process (1st STP), ventral direction (V) and dorsal direction (D). (C) Longitudinal sonogram (10 MHz linear rectal probe, 7 cm depth) in a 5-year-old cow showing the sacroiliac joint (SIJ), iliac wing (IW), first sacral transverse process (1st STP), lateral direction (LAT) and medial direction (MED). Note: A prominent bony lip (arrows) was imaged at the point of junction between the iliac wing and body (IB). (D) Longitudinal sonogram (10 MHz linear rectal probe, 5 cm depth) of the same animal in Figure: 5C showing the ventral aspect of the sacrum (S), ventral sacral foramina (VSF), ventral direction (V) and dorsal direction (D). Proximal is the central portion and Distal is the lateral portion)
DISCUSSION
Early and accurate diagnosis of pelvic injuries in large animals are of great importance from the economic point of view (Grubelnik et al., 2002; Divers and Peek, 2008; Gundelach et al., 2013). In the past, the diagnosis of pelvic affections primarily relied on physical or X-ray examination. The reliability of physical examination in terms of palpation depends mainly upon the skillfulness of the examiner and the ability to detect painfulness, abnormalities, and crepitation. On its own, this method is of little significance, especially in mature and well-muscled animals in which contour change and crepitation are not always noticeable (Dirksen, 1990; Fessl, 1992; Weaver, 1992; Stanek, 1997; Baumgartner, 2000). On the other hand, the application of X-ray for pelvic examination in adult animals is technically difficult (Ferguson, 1997; Farrow, 1999; Starke et al., 2007; Kofler, 2009). Its use is usually limited to confirm some pelvic injuries in cattle calves until a certain age (Nelson and Kneller, 1985).
Ultrasound has been used for several years in pelvic examination and evaluation of human, (Fink et al., 1995; Konermann et al., 2000), dogs (Kresken, 1996), horses, ponies and donkeys (Schmidt, 1989; Shepherd and Pilsworth, 1994; Clegg, 1995; Reef, 1998; Tomlinson et al., 2001; Kersten and Edinger, 2004; Engeli et al., 2006; Whitcomb, 2012; Walker et al., 2012; Head, 2014; Sharshar et al., 2018). For cattle, little attention has been paid to the pelvis (Grubelnik et al., 2002; Starke et al., 2007; Taguchi et al., 2011), although the technique has been used over 20 in the diagnosis of arthritis and tendovaginitis (Kofler and Edinger, 1995; Kotler, 1997). The present study is the first complete description of the transcutaneous and transrectal ultrasonographic examination of the pelvic girdle (bones, joints, tendon, and ligaments) of the adult cow. Only Grubelnik et al. (2002) described some rather than all of the pelvic osseous structures and joints without any reference to pelvic tendons and ligaments.
Pelvic examination was performed after the restraining of the animal in a stanchion under the effect of xylazine HCl tranquilization and caudal posterior epidural analgesia. Restraining of the animals in such a way provided easy and safe examination while reducing the possibilities of rectal tearing by the elimination of animal straining and movement. In this study, a transcutaneous and transrectal examination of the pelvis were carried out based on those described for equine (Kofler, 2009; Walker et al., 2012; Head, 2014; Whitcomb and Vaughan, 2015; Sharshar et al., 2018). In our opinion, this technique may be superior compared to the previous one described in cattle in which Grubelnik et al. (2002) started their examination by localizing the hip joint first from which other osseous structures can be located by moving the probe forward and backward. The later author stated that the obtained results were greatly influenced by the animal’s body size as visualization of the joint is difficult in heavily muscled animals. In this study, we did not experience this difficulty as different components of the pelvis were easily localized and examined in a sequential manner irrespective of the animal’s body size. In such a way, any discontinuities and/or injuries can be easily detected.
Our results concerning the ultrasonographic description of iliac wing, iliac body, ischium, and hip joint correspond with previously published data in cattle (Grubelnik et al., 2002) and equine (Pilsworth, 2003). On the other hand, we have not been able to find any reports in the ultrasonographic literature before this study concerning ultrasonographic descriptions of sacral tuber, tuber coxae, ischial tuber, pelvic tendon, and ligaments. We try to interpret our results in the light of previously published data in horses.
Unlike in equine, sacral tuber and tuber coxae of cow are greatly differed. While, ischial tuber nearly has the same ultrasonographic description as horses and donkeys (Head, 2014; Sharshar et al., 2018). In comparison with equine, sacral tuber appeared larger and slightly irregular with prominent cranial and caudal process. This result coincides with previously published data in the anatomical literature of cattle (Budras and Habel, 2003). The tuber coxae of cow showed a marked irregularity at its mid-region. Such findings required careful evaluation and should not be neither interpreted as splintered fracture nor pathological changes. In our opinion, the anatomical variation of tuber coxae between cow and horse may be important to provide stronger attachment for the abdominal muscles (external and internal abdominal oblique) to tolerate stress from voluminous abdominal contents (rumen and intestine) in ruminant.
According to this study, the proximal part of the first sacral spinous process was located midway between both sacral tubers at the same level or slightly higher near the skin surface. This made its imaging and evaluation easier comparison to equine (Kersten and Edinger, 2004). From our point of view evaluation and comparing the distance between the first sacral spinous process and each sacral tuber is very important to detect left to right asymmetry as well as to give an idea about the status of the sacroiliac joint.
Evaluation of pelvic tendon and ligaments is very important because they may be frequently prone to injuries and tearing as a result of trauma or slipping especially during the breeding season. Our results concerning the ultrasonographic appearance of the dorsal aspect of the dorsal sacroiliac ligament-thoracolumbar fascia combination and the lateral portion of the dorsal sacroiliac ligament are in agreement with previously published data in equines (Engeli et al., 2006; Sharshar et al., 2018). According to this study, the thoracolumbar fascia fused to the medial aspect of the dorsal portion of the dorsal sacroiliac ligament in all examined animals. This configuration is similar to that recorded in donkeys (Sharshar et al., 2018) and the majority of horses (Engeli et al., 2006).
The anatomic configuration of the hip joint in ruminants renders it to be liable for injury when subjected to stress (Greenough et al., 1981). Luxation and fracture of the hip joint are of the most commonly encountered causes of lameness in ruminants (Starke et al., 2007; Taguchi et al., 2011). In this study, localization of the hip joint was accomplished in the same way as described for equine (Walker et al., 2012; Whitcomb and Vaughan, 2015). Our results concerning the ultrasonographic appearance of the hip joint in cow were in accordance with previously published data in cattle (Grubelnik et al., 2002). For a complete evaluation of the hip joint, besides a transcutaneous examination, it should be evaluated transrectally by examination of the obturator foramen and the medial aspect of the acetabulum especially when caudoventral luxation or acetabular fracture were suspected (Taguchi et al., 2011).
Where not all bony surfaces are accessible through the transcutaneous approach, transrectal examination is very important to complete the evaluation of the pelvis. Combining both types of examination allows tracking bony surfaces, and thus, any discontinuity can be easily registered (Grubelnik et al., 2002). Our results concerning the ultrasonographic appearance of the pubis, pubic rim, medial aspect of the iliac body and the ventral aspect of the sacrum agree with previously published data in cattle (Grubelnik et al., 2002).
According to this study, the pelvic symphysis of cow showed the same ultrasonographic picture as described in equine (Whitcomb, 2012; Sharshar et al., 2018). Despite the maturity of the examined animals, pelvic symphysis was easily identified sonographically at the posterior half of the pelvic floor, while, completely disappeared at the anterior half. It required careful evaluation and should not be misdiagnosed as a pelvic floor gap fracture. Clinically, the transrectal examination of the iliac body, pubis, and the sacrum are very important because fracture may lead to collapse of the pelvis and predispose to dystocia (Gundelach et al., 2013).
The sacroiliac joint was easily located by following the medial aspect of the iliac body in a craniodorsal direction (Walker et al., 2012). This study showed a detailed description of the sacroiliac joint of cow compared to the previous one (Grubelnik et al., 2002). According to this study, the shape of the sacroiliac joint in cow is markedly different from that of equine (Kersten and Edinger, 2004; Walker et al., 2012; Sharshar et al., 2018). It is bounded dorsolaterally by the iliac wing, which appeared as a concave hyperechoic arch with prominent bony lip at its lateral end. These results are in parallel with anatomical records by Budras and Habel (2003). In our opinion, it can be presumed that this anatomical variation may provide a greater stability of the sacroiliac joint in cattle compared to equine.
Concerning pelvic measurements evaluated in this study, there is no significant difference between pelvic halves. In our opinion careful and combining the evaluation of different pelvic measurements is very important to asses different causes of left to right pelvic asymmetry.
CONCLUSION
Ultrasonography provides a rapid, efficient and safe method for pelvic anatomical description and evaluation of cow under field conditions. The obtained results may be used as a reference guide for future pelvic evaluation studies of any pathological lesion in cow pelvis.
Acknowledgements
We would like to acknowledge our institution which supported this work. We also would like to thank Dr. Ahmed Mousa, Department of Biochemistry, Faculty of Veterinary Medicine, University of Sadat City, Egypt for his assistance in performing statistical analysis for the collected parameters. We would also like to thank the staff within our department who contributed to the care of the examined animals.
Authors Contribution
All authors contributed in design of the study, analysis and interpretation of the data, drafting the manuscript and the Final Approval of the Completed Article.
Conflict of interest
The authors declare that there is no conflict of interest
References






