Advances in Animal and Veterinary Sciences
Research Article
Synthetic Salicylates and/ or Pioglitazone Ameliorative Effect in Type 2 Induced Diabetic Rats
Yousef M. Shehata1, Amany I. Ahmed1, Nada Y. Nasr1, Ahmed Hamed Arisha2*
1Department of Biochemistry, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; 2Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt.
Abstract | Type-2 diabetes (T2D) is a metabolic disorder characterized by insulin resistance followed by pancreatic islet Beta-cell failure. Inflammation plays a crucial role in both insulin resistance and pancreatic Beta-cell failure. Salicylates and pioglitazone are known to have antidiabetic effect and are used for treatment of type 2 diabetes (T2D). This experiment was designed to investigate the antidiabetic effect of low and high doses of salicylates either alone or in combination with pioglitazone. Seventy male albino rats were randomly divided into seven groups: control, untreated diabetic, diabetic treated with low dose of salicylates (10 mg /kg/day), diabetic treated with high dose of salicylates (120 mg/kg/day), diabetic treated with pioglitazone (10 mg /kg/day), diabetic treated with both low salicylates and pioglitazone and diabetic treated with both high salicylates and pioglitazone. T2D was induced following 12 weeks high fat and high fructose (HFHF) dietary protocol. Both low and high doses of salicylates alone or in combination with pioglitazone ameliorate hyperglycemia, hyperlipidemia, insulin resistance, reduced the level of proinflammatory cytokines and increased adiponectin with activation of PPAR-γ and inhibition of NF-kβ. Co-administration of salicylates and pioglitazone was more effective in improving overall metabolic parameters compared to each of the monotherapy. Collectively, administration of acetylsalicylic acid, even at low doses, has the ability to potentiate the PPAR-γ agonist pioglitazone suggesting a new combination for therapeutic applications.
Keywords | Acetylsalicylic acid, Pioglitazone, Type 2 diabetes, Inflammation, Insulin resistance
Received | September 19, 2019; Accepted | October 26, 2019; Published | December 12, 2019
*Correspondence | Ahmed Hamed Arisha, P.h. D. Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; Email: [email protected]
Citation | Shehata YM, Ahmed AI, Nasr NY, Arisha AH (2019). Synthetic salicylates and/ or pioglitazone ameliorative effect in type 2 induced diabetic rats. Adv. Anim. Vet. Sci. 7(s2): 137-144.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s2.137.144
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Shehata et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is the most common metabolic disorder, characterized by insulin resistance followed by pancreatic islet beta-cell failure leading to hyperglycemia (Asmat et al., 2016). Insulin resistance is defined as a decreased response of peripheral tissues to normal levels of circulating insulin. Insulin resistance is usually compensated by hyperinsulinemia. In these regards, animal models are cornerstone to our ever-advancing knowledge on the pathophysiology of T2D and related complications (King, 2012). Elevated daily consumption of fructose has been recently linked to the type 2 diabetes. Furthermore, high fat high fructose (HFHF) was used to induce T2D (Pyo and Lee, 2014). This HFHF T2D model gives significant inputs about the pathophysiological alterations associated with the early stage of T2D; hyperglycemia, insulin resistance, and abnormal lipid profiles (Samuel, 2011). Additionally, inflammation plays a crucial role in both insulin resistance and pancreatic beta-cell failure (Pechlivani and Ajjan, 2018). T2D is considered as inflammatory disorder characterized by increasing circulating levels of cytokines, chemokines and C-reactive protein (CRP) (Donath and Shoelson, 2011). Increase of insulin signaling impairment in adipocytes; the release of cytokines and chemokines from adipose tissues into the circulation stimulate inflammation in pancreatic islet and other tissues (Donath and Shoelson, 2011). The production of inflammatory molecules associated with insulin resistance such as tumor necrosis factor-alpha (TNF-α), Interleukin-6 (IL-6), IL-1β, and IL-8 stimulate NF-kβ pathway stimulating vicious loop of insulin resistance (Hameed et al., 2015). Adiponectin which is a protective adipokine, acts as anti-inflammatory cytokine and enhances insulin sensitivity (Rehman and Akash, 2016). It inhibits the expression of TNF-α, IL-6 and hepatic gluconeogenesis and stimulates glucose uptake in skeletal muscles and fatty acid oxidation in liver and skeletal muscles. Adiponectin acts as a biomarker for insulin resistance (Park et al., 2015).
Pioglitazone, one of thiazolidinediones (TZDs) or glitazones, is considered insulin sensitizers and therefore used in the treatment of T2D. Pioglitazone has anti-diabetic effect through an increased transactivation of peroxisome proliferator activated receptors-gamma (PPAR-γ agonists), a reduced gluconeogenesis and increase glucose and lipid metabolism (Nanjan et al., 2018). A decreased insulin resistance (Rehman and Akash, 2016), results in an increased glucose uptake through GLUT-4 present in skeletal muscles and adipose tissue (Mirza et al., 2019). On the other hand, Salicylates is one of non-steroidal anti-inflammatory drugs that is widely used for antiplatelet therapy in cardiovascular diseases (Majithia and Bhatt, 2018). Salicylates has antidiabetic effect, improve insulin sensitivity and glucose metabolism through mechanisms that are yet to be further explored (Bellucci et al., 2017; Rehman and Akash, 2016). This work aimed to investigate the biochemical and molecular mechanisms related to the antidiabetic effect of both low and high doses of salicylates as well as pioglitazone, either alone or in combination, in diabetic male albino rats.
MATERIAL AND METHODS
Chemicals and reagents
A synthetic salicylate (acetylsalicylic acid; aspirin) was purchased from Sigma-Aldrich chemical Co., St. Louis. Mo. (USA). Pioglitazone (PIO) was purchased from Hikma Pharmaceuticals, Egypt and stored in the dark at -4ºC. A stock solution of PIO was prepared by dissolving it in dimethyl sulfoxide (DMSO; Sigma-Aldrich). Working solutions of PIO (0, 5, 10, 20 and 40 μmol/L) were prepared by diluting the stock solution in a DMSO medium. The DMSO concentration in the working solutions was < 0.1%. The same concentration of DMSO (< 0.1%) was used in the control group.
Experimental design
Seventy male adult albino rats (150-200g) were acquired from the central animal house of the Faculty of Veterinary Medicine, Zagazig University, Egypt. Rats were acclimatized for 2 weeks under standard laboratory conditions prior to any treatments. Rats were kept at a suitable temperature of 20-25 °C, 12-hour light-dark cycle and free access to food and water. The experimental protocol was approved by the ethics committee of Institutional Animal Care and Use (IACUC) number (ZU-IACUC/2/F/34/2018), Zagazig University, Egypt. After 2 weeks of adaptation, rats were subdivided into 2 groups. The first one served as a normal control group (G1, 10 rats); rats were supplied with a typical rat chow for 16 weeks (full experimental period) in addition to pure water. The other 60 rats were fed high fat and high fructose (HFHF) diet formulated as per previously described composition (El-Naggar and El-Dawy, 2019; Pyo and Lee, 2014).
Rats were monitored weekly for both body weight and fasting blood glucose. At 13 weeks, rats (HFHF dietary group) with blood glucose level less than 200mg/dl were considered non-diabetic and were excluded. Only rats which displayed blood glucose levels more than 200 mg/dl and hyper-insulinemia for 7 days were considered resistant and were further confirmed for insulin resistance (IR) using a homeostasis model assessment (HOMA-Insulin Resistance index; HOMA-IR) (Wilson and Islam, 2012). Diabetic rats in the HFHF induced diabetes were then randomly subdivided into six groups and were given different treatments for the following 4 weeks: group 2 (G2): diabetic received no treatment, group 3 (G3): diabetic and daily treated orally with low-dose salicylates at 10 mg/kg (Patumraj et al., 2000; Pyo and Lee, 2014), group 4 (G4): diabetic and daily treated orally with high-dose salicylates at 120 mg/kg (Yuan et al., 2001), group 5 (G5): diabetic and daily treated orally with pioglitazone at 10 mg/kg (Lee et al., 2007; Schaalan, 2012), group 6 (G6): diabetic and daily treated orally with both low-dose salicylates (10 mg/kg) and pioglitazone (10 mg/kg), group 7 (G7): diabetic and daily treated orally with both high-dose salicylates (120 mg/kg) and pioglitazone (10 mg/kg). The dose was modified weekly in relation to changes in weight gain to sustain analogous dosage per kg body weight of rat throughout the study period.
Sampling
Rats were sacrificed via cervical decapitation, blood was collected, serum and plasma were separated by centrifugation at 3000 rpm for 20 mins and sent for the different biochemical and hormonal assays. A 50 mg of liver tissue was collected and used for gene expression analysis.
Biochemical analysis
Serum glucose was determined by oxidase method using Spectrum Diagnostics glucose kit (Spectrum Diagnostics, Egypt) (El-Gayar et al., 2012). Estimation of serum insulin level by ELISA rat insulin kits using Ray Bio kit (RayBiotech, Inc., USA). Lipid profile was measure using Spectrum diagnostic kit (Spectrum Diagnostics, Egypt) following manufacturer instructions. The direct enzymatic colorimetric liquid method was used for determination of high density lipoprotein -c (HDL-c) and low-density lipoprotein -c (LDL-c) calculated according to friedewald formula (Friedewald et al., 1972). Evaluation of triacylglycerol (TAG) using GPO-PAP enzymatic colorimetric method (Bucolo and David, 1973), cholesterol liquizyme CHOD-PAP (Penttilä et al., 1981) and free fatty acid (FFA) were determined spectrophotometrically by enzymatic colorimetric method using Biovision FZscreen TM (BioVision, Inc., USA). Serum concentrations of TNF-α, IL-6 and adiponectin were determined by the commercially available rat ELISA kits from MyBioSource (MyBioSource, USA) following manufacturer instructions.
Relative quantitative RT-PCR analysis
A detailed description of the protocols used has been previously reported (Arisha et al., 2019; Arisha and Moustafa, 2019; Khamis et al., 2020). The real-time RT-PCR was performed in aMx3005P Real-Time PCR System (Agilent Stratagene, USA) using 5x HOT FIREPol EvaGreen qPCR Mix Plus (Solis Bio Dyne, Tartu, Estonia) and specific primers for the target genes (Table 1). The relative expression of each of the gene normalized to the housekeeping GAPDH was reported as fold change by 2−ΔΔCT (Livak and Schmittgen, 2001).
Statistical analysis
Statistical analysis was carried out using one-way ANOVA (SPSS 16, Chicago, Ill). Data were expressed as Mean±SEM where P< 0.05 was statistically significant.
RESULTS
Effect of administered salicylates and/or pioglitazone on the glycemic index and HOMA-IR
Dietary supplementation HFHF diet induces the development of type 2 diabetes characterized by elevated glucose, abnormal insulin concentrations and insulin resistance compared to the control group (Table 2). However, the plasma glucose and insulin concentrations of the diabetic group treated with salicylates (low and high dose) and/or pioglitazone were significantly (P < 0.01) lower than the diabetic group. The degree of insulin resistance was estimated at the baseline by an insulin score (HOMA-IR). Low HOMA-IR values indicate high insulin sensitivity, whereas high HOMA-IR values indicated low insulin sensitivity. As shown in Table 2, HOMA-IR was significantly (P < 0.01) higher in the HFD group than others but HOMA-IR of the diabetic group supplemented with salicylates (low and high dose) and/or pioglitazone were significantly lower than the diabetic group.
Effect of administered salicylates and/or pioglitazone on the lipid profile and FFA levels
The diabetic group showed significant (P < 0.01) increase in serum levels of FFAs and development of diabetic dyslipidemia that characterized by increase of TC, TAG, LDL-c, VLDL-c and decrease of HDL-c when compared to the normal control group. The HFHF group treated with salicylates (low and high dose) and/or pioglitazone showed significant decrease in serum levels of FFA, TC, TAG, LDL-c and VLDL-c with increase serum levels of HDL-c when compared to the HFHF group.
Effect of administered salicylates and/or pioglitazone on pro inflammatory cytokines and adiponectin levels
The diabetic group showed significant (P < 0.05) increase in serum levels of TNF-α, IL-6 and decrease serum adiponectin level when compared to the normal control group. Diabetic group treated with salicylates (low and high dose) and/or pioglitazone showed significant (P < 0.05) decrease in serum concentration of TNF-α, IL-6 with increase serum levels of adiponectin when compared with HFHF group.
Effect of administered salicylates and/or pioglitazone on hepatic mRNA expression of PPAR- γ and NFKB-p65
Diabetic group showed significant (P < 0.01) decrease in PPAR-γ gene expression with significant (P < 0.001) increase in NF-KB gene expression when compared with normal control group (Figure 1 A&B). Administration of salicylates and/or pioglitazone to diabetic rats showed significant (P < 0.01) increase in PPAR-γ gene expression with significant (P < 0.001) decrease in NF-KB gene expression when compared with diabetic untreated group (Figure 1 A&B).
DISCUSSION
Salicylates has antidiabetic effect by unclear mechanism and its clinical use has been limited due to its adverse effects that are associated with high dosage (Sami et al., 2012). It may act as antidiabetic through inhibition of Ikkβ/NF-kβ and, especially at high dosage, subsequently reduced proinflammatory cytokines (Rehman and Akash, 2016) and plasma free fatty acids (Rumore and Kim, 2010). Also, An et al., 2009. showed that salicylate decrease proinflammatory cytokines and increase PPAR-γ and adiponectin and therefore result in an inhibited interaction between adipocytes and proinflammatory mediators (An et al., 2009). A study suggests that anti-inflammatory effect of NSAIDs may result an activation of PPAR-γ and inhibition of pro-inflammatory gene expression (Landreth and Heneka, 2001). This study provides a great deal of evidence that low dose salicylates alone is an effective drug for diabetes (Abdin et al., 2010). However, the study outcomes contradicts another observation that showed greater impact
Table 1: Sequence of the primers used in the RT-PCR experiments.
| Gene |
Forward primer (5′–3′) |
Reverse primer (5′–3′) |
Accession no | Product size | Reference |
|
NF-kβ p65 |
CAGGACCAGGA ACAGTTCGAA |
CCAGGTTCTG GAAGCTATGGAT |
NM_199267.2 | 150 | |
|
PPAR-ϒ |
CCTGAAGCT CCAAGAATACC |
GATGCTTTA TCCCCACAGAC |
NM_013124.3 | 153 | |
| Gapdh | GGCACAGTCAA GGCTGAGAATG |
ATGGTGGTGA AGACGCCAGTA |
NM_017008.4 | 143 |
Table 2: Means ±SEM of serum levels of fasting blood glucose, insulin, HOMA-IR in rats fed high fat high fructose diet and treated with salicylates and/ or pioglitazone
| Control | Diabetes | Low salicylates | High salicylates | Pioglitazone | Low salicylates+ pioglitazone | High salicylates+pioglitazone | ||
| Glucose (mg/dl) |
92 .80 ± 1.52g |
221.50 ± 2.72a |
190.15 ± 1.20b |
165.40 ± 3.48c |
150.95 ± 0.66d |
144.30 ± 0.57e |
130.40 ± 0.88f |
|
| Insulin (μU/ml) |
10.41 ± 0.15d |
12.13 ± 0.12a |
13.51 ± 0.28b |
13.40 ± 0.25b |
13.33 ± 0.38b |
12.36 ± 0.35bc |
12.00 ± 0.25c |
|
| HOMA-IR |
2.38 ± 0.14e |
6.63 ± 0.30a |
6.34 ± 0.12b |
5.46 ± 0.16b |
4.96 ± 0.42c |
4.40± 0.16d |
3.86 ± 0.26d |
|
abcdefg Means ± SEM at the same row and bearing different superscripts are significantly different at p ≤ 0.05.
Table 3: Means ±SEM of lipid profile (TC-TG-LDL-HDL-VLDL) in rats fed high fat high fructose diet and treated with salicylates and / or pioglitazone.
| Control | Diabetes | Low salicylates | High salicylates | Pioglitazone | Low salicylates+ pioglitazone | High salicylates+ pioglitazone | |
| TC (mg/dl) |
84.33 ± 2.33d |
112.13 ± 1.16a |
98.66 ± 0.88b |
95.33 ± 0.33b |
90.66 ± 0.66c |
86.68 ± 0.65d |
84.00 ± 0.57d |
| TAG (mg/dl) |
140.33 ± 0.88g |
180.00 ± 0.57a |
171.67 ± 0.89b |
167.00 ± 1.15c |
154.33 ± 0.67d |
150.00 ± 0.57e |
144.67 ± 0.33f |
| LDL-c (mg/dl) |
41.266± 2.15cd |
66.33 ± 0.98a |
50.46 ± 1.047b |
43.43 ± 0.23c |
43.80 ± 1.68c |
35.80 ± 1.24d |
29.70 ± 3.75e |
| VLDL-c (mg/dl) |
28.06 ± 0.40g |
36.80 ± 0.57a |
34.33 ± 0.40b |
33.10 ± 0.63c |
30.70 ± 0.33d |
29.70 ± 0.86e |
28.90 ± 0.69f |
| HDL-c (mg/dl) |
15.05 ±0 .57c |
10.02 ±0.53e |
13.66 ±0.33cd |
16.02 ± 2.06bc |
18.50 ± 0.28bc |
20.83 ± 0.45ab |
25.46 ± 3.30a |
| FFA (mmol/l) |
0.510 ± 0.26d |
0.990 ± 0.30a |
0.780 ± 0.24b |
0.773 ± 0.040b |
0.710 ± 0.24b |
0.610 ± 0.84c |
0.576 ± 0.20d |
AbcdefgMeans ± SEM at the same row and bearing different superscripts are significantly different at p ≤ 0.05.
Table 4: Means ±SEM of FFA, adiponectin, TNF-α and IL-6 in rats fed high fat high fructose diet and treated with salicylates and/ or pioglitazone.
| Control | Diabetes | Low salicylates | High salicylates | Pioglitazone | Low salicylates+ pioglitazone | High salicylates + pioglitazone | |
| Adiponectin (ng/ml) |
2.70 ± 0.54d |
1.64 ± 0.44g |
2.05 ± 0.64f |
2.42 ± 0.18e |
3.33 ± 0.43c |
3.77 ± 0.16b |
3.93 ± 0.13a |
|
TNF- α (pg/ml) |
30.60 ± 0.26g |
50.84 ± 0.30a |
39.56 ± 0.12c |
36.83 ± 0.44d |
41.83 ± 0.40b |
33.26 ± 0.24e |
31.96 ± 0.20f |
| IL-6 (pg/ml) |
41.160 ± 0.53d |
48.66 ± 0.69a |
44.733 ± 0.35b |
43.26 ± 0.17c |
45.33 ± 0.14b |
42.30 ± 0.11cd |
41.90 ± 0.25d |
Abcdefg Means ± SEM at the same row and bearing different superscripts are significantly different at p ≤ 0.05.
on fasting blood glucose at a high dose (Sami et al., 2012). Our results showed that low and high doses of salicylates ameliorate the insulin resistance, hyperglycemia, hyperlipidemia and reduce the inflammatory markers providing good glycemic control. These observations are consistent with other several studies including high doses (Hundal et al., 2002; Kim et al., 2001; Sami et al., 2012; Sethi et al., 2011; Sun et al., 2011; van Diepen et al., 2011; Yuan et al., 2001) and low doses (Amiri et al., 2015; Ibrahim, 2018; Lin et al., 2014). In contrast, early studies showed that salicylates result in an increased insulin resistance in diabetic patients although decrease of fasting blood glucose may be due to an increased plasma response to insulin (Bratusch-Marrain et al., 1985) and normal patient (Giugliano et al., 1982). This difference in the effect of salicylates may be due to the difference in the experimental model and species studied (Sami et al., 2012). In another study, where low salicylates were used, no difference in blood glucose level was recorded (Lin et al., 2014). Although Sun et al., 2011 reported that high doses of salicylates did not induce notable reduction in blood glucose level (Sun et al., 2011).
In our study we found that low and high dose salicylates caused a significant decrease in insulin level. Also, other studies showed that low salicylates ameliorate hyperinsulinemia (Amiri et al., 2015; Lin et al., 2014). In addition, another study illustrated that low salicylates caused an increase in serum insulin level that indicate development of insulin resistance although increase of PPAR-γ gene expression in male mice, whereas, in female mice, salicylates reversed glucose intolerance with no difference in insulin level (Zhou et al., 2019). Also, Sun et al., 2011, showed that high dose salicylates ameliorate hyperinsulinemia (Sun et al., 2011). In addition, it was reported that high salicylates caused significant increase of insulin secretion (Sethi et al., 2011). Also, an increase in PPAR-γ, that regulates β-oxidation in male mice, suggesting the development of insulin resistance (Zhou et al., 2019). Another study demonstrated that low dose improve hyperlipidemia via inhibition of NF-kβ (Lin et al., 2014). In contrast, further study showed that moderate and high dose caused an increase of TAG which may be due to short duration of salicylates administration to diabetic rats or to the experimental model or species difference (Sami et al., 2012). Our results showed that low and high salicylates caused inhibition of NF-kβ in a pattern demonstrated previously (Lin et al., 2014).
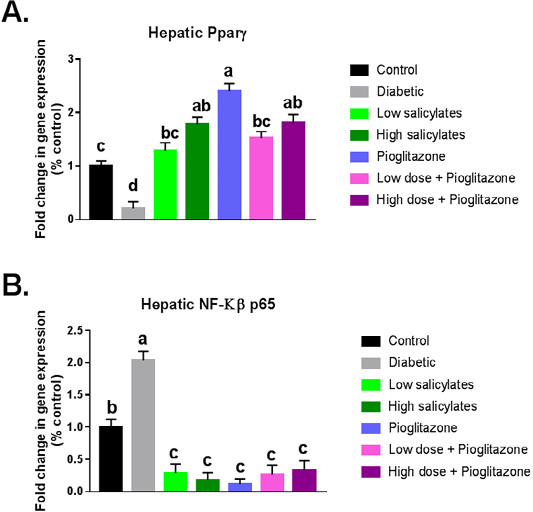
Figure 1: Effect of oral administration of salicylates and/or pioglitazone on hepatic mRNA expression of (a) PPAR-γ and (b) NF-kβp65 in adult male rats. Values are mean±SEM of 8-10 animals per experimental group. Means bearing different superscripts were significantly different at P < 0.05.
Our results showed that low and high salicylates decreased levels of FFA in diabetic rats as described previously using low (Abdin et al., 2010) and high dosages (Sun et al., 2011; Yuan et al., 2001). Furthermore, our results showed that low salicylates decrease levels of TNF-α and IL-6 similar to the one reported previously (Abdin et al., 2010). This is contrary to observation made in another study where low salicylates caused no alteration in TNF-α and IL-6 expression in male mice (Zhou et al., 2019). Our study showed that high salicylates decrease levels of TNF-α, IL-6 (Abdin et al., 2010) and TNF-α in diabetic rats (Sun et al., 2011).
The findings of this study showed that salicylates caused increase in gene expression of PPAR-γ as previously shown (Zhou et al., 2019). Salicylates might be a peroxisome proliferator and may activate PPAR-γ (Lehmann et al., 1997). Also, Fidaleo reported that salicylates might affect the related target gene expression of PPAR-γ in the liver and kidney of rats (Fidaleo et al., 2008). Activation of PPAR-γ may lead to inhibition of NF-kβ (Romeiro et al., 2008; Xue et al., 2010).
TZDs act as anti-inflammatory at higher drug concentration as repression occurs at higher drug concentration than that required for activation (Landreth and Heneka, 2001). Our results showed that pioglitazone improve hyperglycemia and hyperlipidemia and these results are in accordance with several studies (Ding et al., 2005; Refaat et al., 2016; Schaalan, 2012; Vijay et al., 2009). Also, hyperinsulinemia with significant decrease in HOMA-IR and FFA was observed in pioglitazone treated diabetic rats in accordance to previous studies (Refaat et al., 2016; Schaalan, 2012). Also, our study showed that pioglitazone showed inhibition of NF-kβ and increase adiponectin as shown in previous studies (Refaat et al., 2016; Sundararajan et al., 2005; Sung et al., 2006).
Combination of salicylates (low or high) and pioglitazone caused significant decrease in insulin resistance, fasting blood glucose, insulin, FFAs, TNF-α, IL-6, TC, TAG, LDL-c, and VLDL-c and also, caused significant increase of HDL-c and adiponectin providing good glycemic control. This augmented effect can be explained by an observation that both salicylates and pioglitazone can bind and activate PPAR-γ and therefore cumulatively can inhibit NF-kβ. Hence, salicylates has the ability to potentiate effect of pioglitazone or any PPAR-γ agonists and therefore can act alone as antidiabetic drug. Also, some other studies indicate that salicylates and pioglitazone is a good combination simply because pioglitazone potentiates the effects of salicylates by reduction of inflammation and inhibition of platelet function (Evangelista et al., 2005; Mongan et al., 2012). Pioglitazone can suppress the acute gastric mucosal injury in rats that is considered a serious adverse effect of salicylates therapy (Naito et al., 2001). Combination of salicylates and pioglitazone can enhance catalase activity in liver and lead to reduction of oxidative stress, inhibition of non-enzymatic glycation, and cataract formation in type 2 diabetes (Panahi et al., 2019).
Conclusions
Combination of salicylates and pioglitazone provide more beneficial effects, good glycemic control and decrease risk of cardiovascular complications when compared with pioglitazone alone. However, the role of salicylates in insulin resistance requires more basal and clinical studies due to significant difference between experimental studies.
Acknowledgments
The present study was a result of collaboration between the Departments of Biochemistry and Physiology, Faculty of Veterinary Medicine and the Medical Research Center at the Zagazig University, Egypt.
Authors Contribution
A.I.A, N.Y.N and A.H.A conceived the project, researched data, analyzed data and drafted the manuscript. Y.M.S and A.H.A researched the data and reviewed and edited the manuscript. All authors read and approved the final draft of the manuscript.
Conflict of interest
The authors declare no conflicts of interest, financial or otherwise.
References






