Advances in Animal and Veterinary Sciences
Research Article
Testicular Regeneration by Secretome Injection in Cisplatin-Induced Testicular Dysfunction in Rats
Linda Miftakhul Khasanah1, Irma Padeta2, Yosua Kristian Adi3, Teguh Budipitojo2, Yuda Heru Fibrianto4*
1Doctoral Student of Veterinary Science Universitas Gadjah Mada, 2Department of Anatomy, 3Department of Reproduction and Obstetrics, 4Department of Physiology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, 55281.
Abstract | Testicular dysfunction is a degenerative disease characterized by germ cell death during the process of spermatogenesis. The secretome, or mesenchymal stem cell-conditioned medium, can promote tissue regeneration because it contains biological substances such as growth factors and cytokines. Based on a previous study, the secretome could increase the number and motility of sperm and promote the recovery of seminiferous tubule cytoskeletal structure in the testes of rats with testicular dysfunction. A histochemical study on lectin in rats with cisplatin-induced testicular dysfunction treated with secretome has not yet been reported. All animal experiments were divided them into three groups: Group A (low-dose, 0.2 ml/kg body weight (BW) secretome-treated group); Group B (high-dose, 0.5 ml/kg BW secretome-treated group), and Group C (control group). In groups A and B, testicular dysfunction was induced by injection of cisplatin and injected with secretome. The intensities of lectin histochemical staining tended to increase every week after secretome injection. In the high-dose secretome group, the intensity tended to be stronger than in the low-dose secretome group. The results of this study showed that secretome injection improves the regeneration processes in rats with testicular dysfunction, and the higher dose of secretome injection the better the outcome.
Keywords | Secretome, Cisplatin, Testicular dysfunction, Lectin histochemistry, Regeneration
Received | July 30, 2019; Accepted | August 01, 2019; Published | December 08, 2019
*Correspondence | Yuda Heru Fibrianto, Department of Physiology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, 55281; Email: [email protected]
Citation | Khasanah LM, Padeta I, Adi YK, Budipitojo T, Fibrianto YH (2020). Testicular regeneration by secretome injection in cisplatin-induced testicular dysfunction in rats. Adv. Anim. Vet. Sci. 8(1): 11-17.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.1.11.17
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Fibrianto et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cisplatin is one of the chemotherapy drugs that have been used successfully to treat many types of cancer, including soft-tissue, bone, muscle, and blood vessel cancers (Florea and Busselberg, 2011). However, although cisplatin is a potential anticancer drug, it might be toxic to some organ systems, such as the reproductive organs (Amin et al., 2012; Beytur et al., 2012; Reddy et al., 2016). Testicular dysfunction is a degenerative disease and the most common side effect of cisplatin treatment (Ateşşahin et al., 2006; Simsek et al., 2016). Cisplatin initiates the formation of reactive oxidative species, thereby causing oxidative stress in the tissues. Oxidative stress causes germ cell losses and abnormalities (Reddy et al., 2016; Simsek et al., 2016). Cisplatin treatment can reduce spermatozoa production, viability, and motility and increase the number of abnormal spermatozoa (Reddy et al., 2016). Moreover, it can reduce fertility by causing impaired spermatogenesis, chromosomal abnormalities in sperm, and azoospermia (Simsek et al., 2016; Schrader et al., 2002). Previous studies reported that many substances can repair cisplatin-induced testicular dysfunction, such as selenium (Simsek et al., 2016), curcumin (Ilbey et al., 2009), exogenous testosterone (Aminsharifi et al., 2010), antioxidants (Ateşşahin et al., 2006; Surendran et al., 2012; Salem et al., 2012; Prieto and Fuchs, 2009), fish oil (Ciftci et al., 2014), and the secretome (Prihatno et al., 2018).
The secretome, or microvesicles, also known as the exosome, is a factor secreted by stem cells and can be found in stem cell culture medium (Pawitan, 2014). The secretome consists of growth factors and cytokines that play roles as regenerative agents. Growth factors secreted by stem cells into the culture medium are potential reparative agents acting through a paracrine signaling mechanism (Padeta et al., 2017). The secretome plays a role in the coordination of many biological functions such as growth, division, differentiation, apoptosis, and paracrine signaling of the organ (Makridakis et al., 2013). The secretome supports immunomodulatory or anti-inflammatory mechanisms; neoangiogenic processes; and cell survival, differentiation, and recruitment (Farina et al., 2011; Roche et al., 2013). Based on a previous study, the secretome could increase the number and motility of sperm and improve seminiferous tubule cytoskeletal structure in testicular dysfunction (Prihatno et al., 2018). However, studies on the effect of the secretome on the recovery of spermatogenesis in testicular dysfunction rats, employing lectin histochemistry, have not yet been conducted. Therefore, we aimed to investigate the effect of secretome injection on testicular regeneration in cisplatin-induced testicular dysfunction rats.
Materials and Methods
Ethical Approval
This study was approved by the Ethical Committee of Universitas Gadjah Mada with number 00035/04/LPPT/V/2017.
Experimental Animals
All animal experiments were divided randomly into three groups, as described by Prihatno et al. (2018): a low-dose, 0.2 ml/kg BW secretome-treated group (group A); a high-dose, 0.5 ml/kg BW secretome-treated group (group B) and control group (group C). Groups A and B were injected intraperitoneally with cisplatin (PT Dankos Farma, Kalbe Company, Indonesia) at a dose of 3 mg/kg BW three times at 3-day intervals to induce testicular dysfunction, as described by Prihatno et al. (2018) and Reddy et al. (2016). Secretome was injected after testicular dysfunction had developed. Group A and B were injected with secretome four times at 1-week intervals. The healthy control group was not given any treatment. Three rats from each group were sacrificed once weekly, 1 week after secretome injection. Testicular tissues were fixed in Bouin’s solution for 24 hours, embedded in paraffin, sectioned to a thickness of 5 µm, and stained with a lectin histochemical method to visualize the reactivity of Pisum sativum agglutinin (PSA), Lens culinaris agglutinin (LCA), Phaseolus vulgaris leucoagglutinin (PHA-L), and Sophora japonica agglutinin (SJA).
Lectin Histochemical Staining
Lectin histochemistry was performed as described previously (Dorsch et al., 2019; Diaz et al., 2017). Slides were deparaffinized with xylene and rehydrated in a graded ethanol series. Slides were blocked by incubation in 3% H2O2 in methanol for 30 min at room temperature and washed with PBS. Lectin reactivity was visualized with the Starr Trek Universal HRP Detection System Kit (Biocare Medical, USA). Slides were incubated with background Sniper solution for 30 min to block nonspecific proteins. Then, slides were incubated with biotinylated lectin overnight at 4°C. Trekkie Universal Link solution was applied for 30 min, and then Trek Avidin-HRP solution was applied for 20 min. Sites of reactivity were visualized with 3,3’-Diaminobenzidine (DAB) Chromogen. Slides were incubated with Harris hematoxylin counterstain. Then, slides were dehydrated, cleared, and mounted.
Statistical Analysis
All the results were analyzed statistically by Kruskal Wallis test and continued by Mann Whitney test. In this study values p<0,05 was considered as significant.
Results
The PSA lectin reactivities in the control group are shown in spermatogonia, spermatocytes, and spermatozoa (Table 1). In the low-dose secretome-treated group, PSA lectin reactivities were nearly negligible 1 week after the first injection, but the intensity increased 1 week after the second and third injections (Figure 1). By 1 week after the fourth injection, no samples could be analyzed.
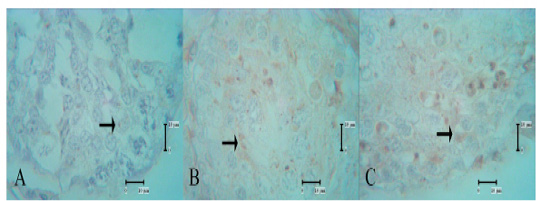
Figure 1: PSA lectin reactivities in the low-dose secretome-treated group; reactivity was not present 1 week after the first injection, but the intensity was increased 1 week after the second and third injections. A: low-dose secretome-treated group 1 week after the first injection, B: one week after the second injection, C: one week after the third injection, arrow: lectin reactivities.
PSA lectin reactivities in the high-dose secretome-treated group were present from 1 week after the first secretome injection. Indeed, the intensity of reactivity in spermatids and spermatozoa increased every week. Very strong reactivity in spermatids was seen 1 week after the fourth high-dose secretome injection (Figure 2).
It was observed that one week after second and third injection significantly differences compared to one week after first injection (p<0,05) in low-dose and high-dose secret-
Table 1: Lectin labeling sites in testis cells
| Spermatogenic cells | PSA | LCA | PHA-L | SJA | ||||||||||||||||
|
DO 1 |
DO 2 |
DO 3 |
DO 4 |
DO 1 |
DO 2 |
DO 3 |
DO 4 |
DO 1 |
DO 2 |
DO 3 |
DO 4 |
DO 1 |
DO 2 |
DO 3 |
DO 4 |
|||||
| Spg | A |
- Aa |
+ Ba |
+ Ba |
n |
- Aa |
- Aa |
++ Ba |
n |
- Aa |
- Aa |
+ Ba |
n |
- Aa |
- Aa |
- Aa |
n | |||
| B |
- Aa |
+ Ba |
+ Ba |
+ a |
- Aa |
- Aa |
+++ Bb |
+++ a |
- Aa |
- Aa |
+ Ba |
+ a |
- Aa |
- Aa |
- Aa |
- a |
||||
| C |
+++ Ab |
+++ Ab |
+++ Ab |
+++ b |
- Aa |
- Aa |
- Ac |
- b |
- Aa |
- Aa |
- Ab |
- b |
- Aa |
- Aa |
- Aa |
- a |
||||
| Spc | A |
+ Aa |
+ Aa |
++ Ba |
n |
- Aa |
+ Ba |
+ Ba |
n |
- Aa |
- Aa |
++ Ba |
n |
- Aa |
- Aa |
- Aa |
n | |||
| B |
+ Aa |
+ Aa |
+ Ab |
+ a |
- Aa |
+ Ba |
++ Cb |
++++ a |
- Aa |
- Aa |
+ Bb |
+ a |
- Aa |
- Aa |
- Aa |
- a |
||||
| C |
- Ab |
- Ab |
- Ac |
- b |
++ Ab |
++ Ab |
++ Ab |
++ b |
+ Ab |
+ Ab |
+ Ab |
+ a |
- Aa |
- Aa |
- Aa |
- a |
||||
| Spd | A |
- Aa |
++ Ba |
++ Ba |
n |
+ Aa |
++ Ba |
++ Ba |
n |
- Aa |
+ Ba |
++ Ca |
n |
- Aa |
++ Ba |
+++ Ca |
n | |||
| B |
+ Ab |
++ Ba |
+++ Cb |
++++ a |
+ Aa |
++ Ba |
+++ Cb |
++ a |
- Aa |
+++ Bb |
++ Ca |
+++ a |
+ Ab |
+++ Bb |
+++ Ba |
+++ a |
||||
| C |
+ Ab |
+ Ab |
+ Ac |
+ b |
+++ Ab |
+++ Ab |
+++ Ab |
+++ b |
+ Ab |
+ Aa |
+ Ab |
+ b |
+++ Ac |
+++ Ab |
+++ Aa |
+++ a |
||||
| Spz |
A |
- Aa |
++ Ba |
+ Ca |
N |
+ Aa |
+ Aa |
+ Aa |
n |
- Aa |
- Aa |
- Aa |
n |
- Aa |
+ Ba |
+ Ba |
n | |||
| B |
+ Ab |
++ Ba |
++ Bb |
+++ a |
+++ Ab |
+ Ba |
+++ Ab |
+++ a |
- Aa |
+ Bb |
+ Bb |
++ a |
- Aa |
+ Ba |
+ Ba |
+ a |
||||
| C |
+ Ab |
+ Ab |
+ Aa |
+ b |
++ Ac |
++ Ab |
++ Ac |
++ b |
- Aa |
- Aa |
- Aa |
- b |
+ Ab |
+ Aa |
+ Aa |
+ a |
||||
| Sc | A |
- Aa |
++ Ba |
+ Ca |
n |
- Aa |
+ Ba |
+ Bb |
n |
- Aa |
+ Ba |
++ Ca |
n |
- Aa |
+ Ba |
+ Ba |
N | |||
| B |
- Aa |
++ Ba |
+++ Cb |
++ a |
- Aa |
+ Ba |
+++ Cb |
+++ a |
- Aa |
+ Ba |
+ Bb |
+ a |
- Aa |
+ Ba |
+ Ba |
+ a |
||||
| C |
- Aa |
- Ab |
- Ac |
- b |
++ Aa |
++ Ab |
++ Ac |
++ b |
++ Ab |
++ Ab |
++ Aa |
++ b |
+ Ab |
+ Aa |
+ Aa |
+ a |
||||
Spg: spermatogonia, Spc: spermatocytes, Spd: spermatids, Spz: spermatozoa, Sc: Sertoli cells, A: low-dose secretome-treated group dose 0.2 ml/kg BW, B: high-dose secretome-treated group dose 0.5 ml/kg BW, C: control group, DO1: one week after the first injection, DO2: one week after the second injection, DO3: one week after the third injection, DO4: one week after the fourth injection, Cp: caput, Cd: cauda. Intensity of labeling: -negative; + weak; ++ moderate; +++ strong; ++++ very strong; n: no data. Uppercase superscript (ABC): statistical for the differences between weeks after injection. Lowercase superscript (abc): statistical for the differences among group. Different superscript was considered as significant (p < 0,05).
ome treated group. Injection of low-dose and high-dose secretome resulted in significant increase the PSA lectin reactivities compared to control group in one week after second and third injection. Administration high-dose secretome resulted in significant increase the PSA lectin reactivities compared to the control group in one week after fourth injection (Table 1).
LCA lectin reactivity was shown in spermatocyte, spermatid, spermatozoa, and Sertoli cells (Table 1). In the control group, there was no reactivity in spermatogonia. One week after the third and fourth injections, reactivities of moderate and strong intensity were evident in the low-dose and high-dose secretome-treated groups, respectively. Reactivities of LCA lectin in spermatocytes at 1 week after the first injection were not shown, but at one week after the second, third, and fourth injections, the reactivities increased in both low- and high-dose secretome-treated groups (Figure 3). The intensity of LCA lectin reactivities in spermatid, spermatozoa and Sertoli cells increased every week. The s-
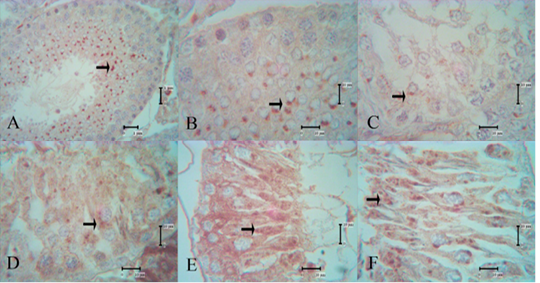
Figure 2: PSA lectin reactivities in the control group and high-dose secretome-treated group; the reaction intensity of spermatids and spermatozoa increased every week, and the strongest intensity were shown 1 week after the fourth injection. A and B: control group, C: high-dose secretome-treated group 1 week after the first injection, D: one week after the second injection, E: one week after the third injection, F: one week after the fourth injection, arrow: lectin reactivities.
trongest intensity of LCA lectin reactivity was in the high-dose secretome-treated group at 1 week after the fourth injection.
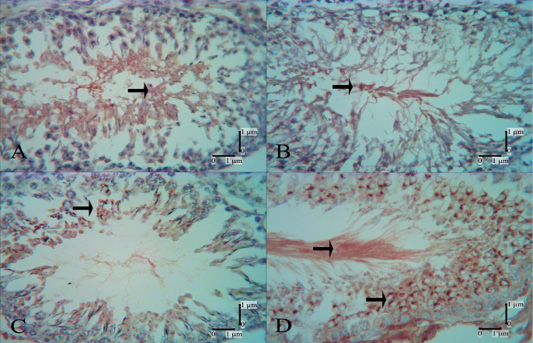
Figure 3: LCA lectin reactivities in the control group and secretome-treated group; no reactivity was evident in spermatocytes in the secretome-treated group 1 week after the first injection (B), but at 1 week after the second injection (C), moderate reactivity was shown. In the secretome-treated group, 1 week after the fourth injection (D) very strong reactivities were evident in spermatocytes and spermatozoa. A: control group, B: low-dose secretome-treated group 1 week after the first injection, C: low-dose secretome-treated group 1 week after the second injection, D: high-dose secretome-treated group 1 week after the fourth injection, arrow: lectin reactivities.
Injection of secretome resulted in significant increase the LCA lectin reactivities in spermatocytes, spermatids, and spermatozoa compared to control group in one week after first injection. It was observed that secretome treated group significantly differences compared to control group (p<0,05) in one week after second and third injection. Administration high-dose secretome resulted in significant increase the LCA lectin reactivities compared to the control group in one week after fourth injection (Table 1).
The PHA-L lectin reactivities in the control group are shown in spermatocytes, spermatids, Sertoli cells and spermatozoa (Figure 4A). In the low-dose secretome-treated group, reactivity was not present at 1 week after the first injection (Figure 4B), but present at 1 week after the second injection in spermatids and Sertoli cells (Figure 4C), and increased in 1 week after the third injection. In the high-dose secretome-treated group, 1 week after the second, third, and fourth injections, the intensity of lectin reactivities increased over those of previous weeks. Very strong reactivities in spermatids were evident in the high-dose secretome-treated group 1 week after the fourth injection (Figure 4D).
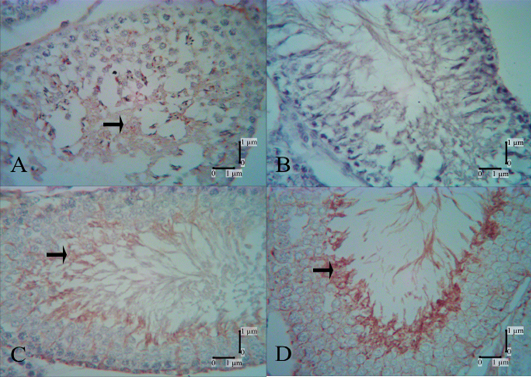
Figure 4: PHA-L lectin reactivities in the control and secretome-treated groups. Reactivity of PHA-L lectin was not shown in the low-dose group 1 week after the first injection (B) but was shown 1 week after the second injection (C); strong reactivities were shown in spermatids in the high-dose secretome-treated group 1 week after the fourth injection. A: control group, B: low-dose secretome-treated group 1 week after the first injection, C: low-dose secretome-treated group 1 week after the second injection, D: high-dose secretome-treated group 1 week after the fourth injection, arrow: lectin reactivities.
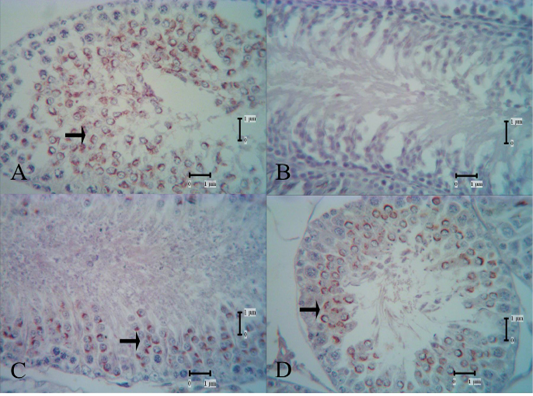
Figure 5: SJA lectin reactivities in the control group and secretome-treated group; reactivity of SJA lectin was not shown in the low-dose group 1 week after the first injection (B). In the high-dose secretome-treated group 1 week after the second and third injection, the reactivities shown were moderate to strong. A: control group, B: low-dose secretome-treated group 1 week after the first injection, C: high-dose secretome-treated group 1 week after the second injection, D: high-dose secretome-treated group 1 week after the third injection, arrow: lectin reactivities.
It was observed that one week after second and third injection significantly differences compared to one week after first injection (p<0,05) in low-dose and high-dose secretome treated group. Administration high-dose secretome resulted in significant increase the PHA-L lectin reactivities compared to the control group in one week after second injection. Injection of secretome resulted in significantly differences compared to control group in one week after third injection in spermatogonia, spermatocyte, and spermatids.
SJA lectin reactivity in the control group was evident in spermatids, Sertoli cells, and spermatozoa (Table 1). In the low-dose secretome-treated group, SJA lectin reactivities were seen 1 week after the second injection, and the intensity increased 1 week after the third injection (Figure 5). One week after the first injection, there was no reactivity and week reactivity in spermatids of the low-dose and high-dose secretome-treated group, respectively. Strong reactivity was shown in spermatids 1 week after the second high-dose secretome injection, but in the low-dose secretome-treated group, strong reactivity was only reached one week after the third injection. It was observed that one week after second injection significantly differences compared to one week after first injection (p<0,05) in low-dose and high-dose secretome treated group in spermatids and Sertoli cells. Administration secretome resulted in significant increase the SJA lectin reactivities compared to the control group in one week after first and second injection in spermatids.
Discussion
Cisplatin causes parenchymal atrophy in testicular tissues (Ateşşahin et al., 2006). Cisplatin causes increases in the numbers of dead and morphologically abnormal sperm (Rezvanfar et al., 2013). Cisplatin induces testicular dysfunction by breaking DNA chains and disruption the cell cycle, resulting in damage to spermatogenic cells, Sertoli cells, and Leydig cells. Apoptosis of germ cells and loss of the junction between spermatogenic cells and Sertoli cells may causes changes in lectin reactivity (Manesh et al., 2017).
One week after secretome injection, the appearance of spermatogenic cells improved, and all lectin reactivities appeared weak in both low- and high-dose groups. One week after the second injection, PSA, LCA, and SJA lectin reactivity was moderate, but that of PHA-L lectin was weak. It was observed that secretome treated group significantly differences compared to control group (p<0,05) in one week after second and third injection. Lectin reactivities increased every week, and the strongest reactivity was detected 1 week after the fourth injection. Administration high-dose secretome resulted in significant increase the lectin reactivities compared to the control group (p<0,05) in one week after fourth injection. The intensity of labeling with lectin in the high-dose secretome group was stronger than that in the low-dose secretome group. This may indicate that a higher dose of secretome can improve the regenerative process to a greater extent. These data showed that the secretome could stimulate cell proliferation and promote recovery of testicular cells. The regenerative process is characterized by the presence of sperm and spermatogenesis. In our previous study, the secretome could increase the number and motility of spermatozoa in cisplatin-induced testicular dysfunction rats (Prihatno et al., 2018).
Modifications in lectin or glycoprotein expression on the sperm surface have been shown to occur during sperm formation, maturation, capacitation, and the acrosome reaction in some species (Nicolson et al., 1977; Schwan and Kohler, 1979; Pastor et al., 2003). Lectins have a specific binding affinity for glucose residues and have been used to investigate the distribution of glycoproteins in various tissue, including testes (Abd-Elmaksoud et al., 2008), during cell differentiation and maturation (Spicer and Schulte, 1992). Lectin histochemistry is a valuable method of determining changes in the glycoconjugate content during spermatogenesis under normal and pathological conditions (Abd-Elmaksoud et al., 2008). Lectins are proteins that recognize and bind to carbohydrate complexes in glycolipids and glycoproteins (Ghazarian et al., 2011).
Glycoconjugates play roles in cell differentiation, maturation, recognition, adhesion, and cell interaction (Agungpriyono et al., 2007), in addition to the exchange of substrates between the two cell types (Pastor et al., 2003). PSA and LCA lectin reactivities indicate the presence of mannose residues, which are the result of metabolism. Mannose residues have a role in the transport of ions (Spicer and Schulte, 1992; Pastor et al., 2003), which are essential nutrients in sperm development (Klein and Cherrington, 2014). The results of our research are congruent with the reports stating that the PSA and LCA lectin reactivities were seen in Sertoli cells that have a role in nourishing of the germ cells. The PSA lectin reactivities detected in Sertoli cells, which secrete various paracrine factors involved in the control of germ cells, peritubular cells, and Leydig cells, also facilitate spermatogenesis. PHA-L lectin reactivity indicates the presence of galactose residues. Galactose residues have a role in cell adhesion and are markers of cell differentiation (Abd-Elmaksoud et al., 2008). SJA lectin reactivity indicates the presence of acetylglucosamine residues, which regulate membrane interactions and membrane permeability (Abd-Elmaksoud et al., 2008) and are selectively expressed in the final stage of spermatogenesis (Lee and Damjanov, 1984). The results of our study are congruent with the theory that PHA-L and SJA lectin reactivities are shown in spermatids that are ready to differentiate into spermatozoa. Leydig cells of hamsters contain glycans with fucosyl, mannosyl, glucosyl, neuraminic acid, and galactosyl residues, which carry out structural and transport functions and participate in androgen synthesis and cell regulation (Pinart et al., 2002). The presence of glycoconjugates in the secretome-treated group may indicate improvement of spermatogenic cell health and the steroidogenesis process, also an indicator of good spermatogenesis.
Conclusion
The secretome could improve the regeneration processes by repairing testicular cells and restoring spermatogenesis in rats with testicular dysfunction characterized by the presence of lectin reactivity in spermatogenic cells and spermatozoa.
Acknowledgements
Authors would like to thank the Ministry of Research, Technology and Higher Education of Indonesia for fully funding this study (Grant number: 5724/UN1.DITLIT/DIT-LIT/LT/2018). Authors would also like to thank the Department of Anatomy and Laboratory of Microanatomy that supported and facilitated this study.
Conflict of interest
The authors declare that they have no Conflict of interest.
Authors Contribution
Linda Miftakhul Khasanah developed the concepts and designed the experiments, visualized lectin reactivity by using lectin histochemical method and wrote the manuscript. Irma Padeta carried out euthanized rats, collected testes and fixed sample in Bouin’s solution, processed testicle tissues for paraffin-embedded method and read and approved the final manuscript. Yosua Kristian Adi created testicular dysfunction in rat with Cisplatin and treated rats with secretome and read and approved the final manuscript. Teguh Budipitojo developed the concepts and designed the experiments, Data analysed and interpreted the data also read and approved the final manuscript. Yuda Heru Fibrianto developed the concepts and designed the experiment Secretome production and read and approved the final manuscript.
References






