Advances in Animal and Veterinary Sciences
Research Article
Stannius Corpuscles in African Catfish (Clarias gariepinus): Histological and Ultrastructure Studies
Mayada Wahid Karkit1*, Yaser Hosny Ali Elewa1, 2*, Hoda Foad Salem1, Mohammad Hafez Bareedy1
1Department of Histology and Cytology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt; 2Laboratory of Anatomy, Department of Biomedical Sciences, Graduate School of Veterinary Medicine, Hokkaido University, Kita 18, Nishi 9, Kita-Ku, Sapporo, Hokkaido 060-0818, Japan.
Abstract | Stannius corpuscles (SC) are unique endocrine gland in the kidney of bony fish. It plays an important role in regulating calcium haemostasis via secretion of hypocalcine (stanniocalcin) hormone. The structure of Stannius corpuscles has been clarified in several fish species; however, little was elucidated concerning that in African catfish (Clarias gariepinus). Therefore, the aim of the current study is to reveal the structural characteristics of SC in African catfish (Clarias gariepinus) among both sexes. Histological and ultrastructural examinations of SC were revealed among both sexes. The SC were paired, oval white colored bodies embedded in the trunk area of posterior kidneys. Histologically, the SC were surrounded by thin connective tissue (C.T) capsule from which many septa were extended dividing the glands into incomplete lobules. The parenchymal cells consist of two types; one with intensely eosinophilic cytoplasm and the other were weakly stained. Interestingly, the proportion of cells was varied among male and female. The female showed significant higher percentage of lightly eosinophilic cells than the intensely stained cells. On the other hand, the predominant cell type in male was the intensely stained one. Transmission electron microscopic observations revealed the presence of two types of cells; predominant type I (electron dense and electron lucent cells) and type II. The former showed more secretory granules than the later. In conclusion, our results revealed sexual differences in the proportion of cells and suggested that the type I could be responsible for hypocalcine hormone secretion. However, further investigations are required to reveal the structure of SC among different seasons.
Keywords | Corpuscles of Stannius, African catfish, Clarias gariepinus, Histology, Ultrastructure
Received | September 11, 2019; Accepted | October 06, 2019; Published | October 10, 2019
*Correspondence | Yaser Hosny Ali Elewa and Mayada Wahid Karkit, Department of Histology and Cytology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt; Email: [email protected]; [email protected]
Citation | Karkit MW, Elewa YHA, Salem HF, Bareedy MH (2019). Stannius corpuscles in african catfish (Clarias gariepinus): Histological and ultrastructure studies. Adv. Anim. Vet. Sci. 7(s2): 6-11.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s2.6.11
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Karkit et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The African catfish (Clarias gariepinus) are freshwater fish. It considered as one of the most highly significant valued species in Egypt. The African catfish are superior to other species because they can resist a wide range of habitant conditions; including hypoxia, extreme temperatures, adaptation to poor quality water, and coexistence with bad food; while achieving high growth rates. All physiological processes in fish are subject to the dominance of hormones produced by the endocrine glands. In fish, several endocrine glands have been reported including the pituitary gland, islets of Langerhans (Brockmann body), thyroid gland, adrenal gland, and the corpuscles of Stannius (Stannius corpuscles) (SC) (Gu et al., 2015; Prasad et al., 2017).
The Stannius corpuscles (SC) are considered as unique endocrine gland in bony fish, and have not been identified in higher vertebrates (Gu et al., 2015; Prasad et al., 2017). The SC in fish are exclusively responsible for the secretion of homodimeric glycoprotein hormone known as hypocalcine which is directly in charge of maintaining the balance between calcium and phosphate via gills, gut, and kidneys (Wagner and Dimattia, 2006). It co-reduces the absorption of calcium from the surrounding water through the gills and digestive tract (Ahmad et al., 2002). The ultimobranchial glands as well as the Stannius corpuscles are hypocalcemic factors (Chakrabarti and Mukherjee, 1993).
The SC are found as a sac-like organ in the kidney either completely or partly embedded whose location and number varies among species (Kaneko et al., 1992; Pandey and Pandey, 2013). Several studies have been reported the structure of SC in seawater fish (Bedjargi and Kulkarni, 2014b) however, little was done concerning fresh water fish specially that in African catfish (Bedjargi and Kulkarni, 2014a). Therefore, in the present study, detailed light structure and ultrastructure of SC of Clarias gariepinus has been presented with special focus on the sexual dimorphism.
MATERIALS AND METHODS
Animals and ethics statement
Adult eight healthy freshwater teleost Clarias gariepinus of both sexes (4 males and 4 females) were collected locally from Moyes canal, Zagazig city, Egypt. The weight of each fish was between 400 to 600 gm. The SC was directly extracted from the kidney tissue after scarifying each fish. The guide for care and use of laboratory animals including fish at Zagazig University, Faculty of Veterinary medicine was under the number; ZU-IACUC/2/F/116/2018.
Histological examination
The collected tissues were directly fixed in Bouin’s fluid overnight followed by washing and processing. Briefly, the specimens were subjected to ascending grades of ethanol (70%, 80%, 95% and absolute) for dehydration, then were clarified in xylene and placed in paraffin wax. Finally, 4 µm paraffin sections were obtained and stained with hematoxylin and eosin (H and E) stain which were photographed via a digital Dsc-W 800 super steady cyper shot camera connected to an Olympus BX 21 light microscope at Histology and Cytology Department, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Ultrastructural Examination
The SC were removed rapidly after fish slaughtering and then fixed with 3% glutaraldehyde in 0.1 M phosphate buffer (PB) (pH 7.4) for 2 hours then followed by post-fixation with 1% osmium tetroxide (OsO4) in 0.1 M PB for 1 hour. After that, the samples were dehydrated in ascending grades of ethyl alcohol, and embedded in epoxy resin. Semithin sections were prepared using a MT2 Sorvall ultramicrotome and stained with toluidine blue. Ultrathin sections (70 nm) were double stained with uranyl acetate and lead citrate then were photographically captured using a (JOEL, JEM-21000) transmission electron microscope at the Faculty of Agriculture, Mansoura University, Egypt.
Morphometrical and statistical analysis
Using an image J analysis software (Fiji image j; 1.51 n, NIH, USA) and represented capture micrographs from HandE stained sections at 400 X magnification, the number of both lightly and intensely stained cells were counted in both sexes and the ratio of both cells to the total cells were compared among both sexes. Statistical analysis was performed using MS Excel 2016. Numerical data were expressed as median ± interquartile range. Differences between the ratio of both cells and between both sexes were tested using the chi-square test. A p-value < 0.05 was considered statistically significant, n=4 for each sex.
RESULTS
Anatomically, the SC of African catfish was partially embedded in the posterior regions of trunk kidneys (Figure 1A). The SC consist of paired of small, oval, colored white or creamy bodies (Figure 1B).
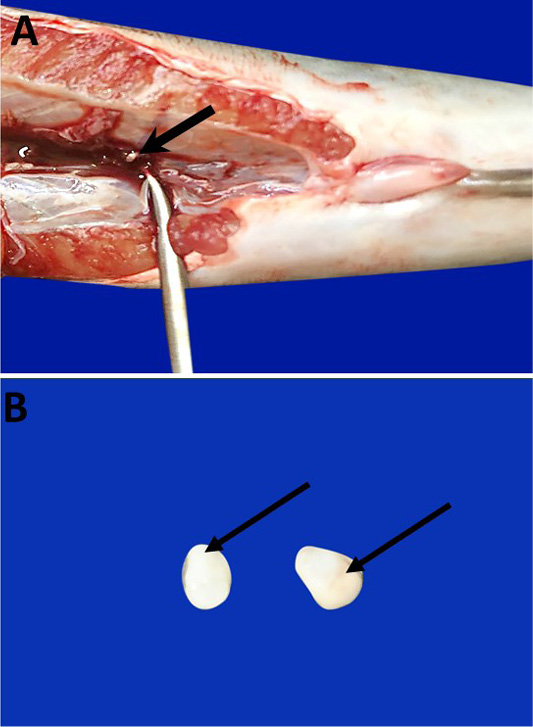
Figure 1: Photomacrographs show Catfish SC which are partially embedded in the posterior region of trunk kidney (bold arrow) (A). They are paired of small, oval, colored white or creamy bodies (arrows) (B).
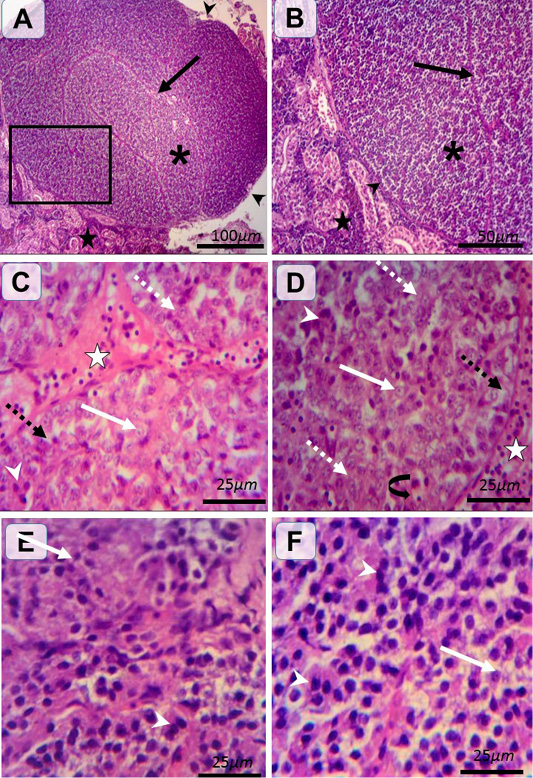
Figure 2: Histological structure of female and male catfishes SC. Representative photomicrographs of HandE stained sections illustrating renal tissues (black stars), C.T. capsule (black arrow heads), septa (black arrows), SC of incomplete lobules (asterisk) (A and B). Type I (weakly stained) cells are rounded-shaped with pale homogenous eosinophilic (white dashed arrows) or vacuolated (black dashed arrows) cytoplasm and lightly stained basophilic rounded nuclei with prominent nucleoli (white arrows). Type II (intensely stained) cells are irregular-shaped with deeply eosinophilic cytoplasm and darkly stained basophilic rounded nuclei (white arrow heads) (C, D, E andF). The pronounced cells are type I in female (C andD) and type II in male (E andF). Notice the C.T. septa housing blood vessels (white star), plentiful blood capillaries within gland (closed arrow).
Histologically, the SC are associated with renal tissues at which thin connective tissue (C.T.) capsule was observed around the gland and separate the gland from the surrounding renal tissues. From the capsule, the highly vascularized C.T. septa were extended into the glandular tissues parenchyma dividing it into incomplete lobules (Figure 2A and B). The SC were richly vascularized. According to H and E staining affinity of Parenchymal cells, cytoplasm and nuclei, cells were categorized into two types; type I and type II. Type I (weakly stained) cells were large and rounded-shaped cells which manifested by pale homogenous eosinophilic or vacuolated cytoplasm and lightly stained basophilic rounded nuclei with prominent nucleoli. Type II (intensely stained) cells were small and irregular-shaped cells, which characterized by deeply eosinophilic cytoplasm and darkly stained basophilic rounded nuclei. Type I cells were arranged in cords and sometimes in clusters or follicles however, type II cells were mainly arranged in clusters. Interestingly in female, type I cells were more predominant than type II (Figure 2C and D). On the other hand, type II cells were more pronounced than type I in male (Figure 2E and F). Statistical analysis showed significant differences in the proportion of the two types (weakly and intensely stained) cells among both sexes. Furthermore, significant differences were observed between the two types of cells in the same sex as shown in (Figure 3).
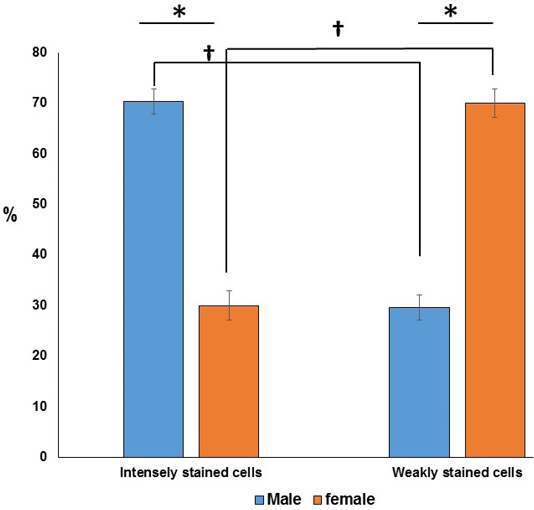
Figure 3: Chart showing (*) significant differences in the ratio of the two cell types in the same sex. Furthermore, (†) significant differences are observed between the two types of cells in between both sexes.
Ultrastructural investigations were done in both sexes (Figure 4) at which the septa were well vascularized. Type I and II glandular cells were mainly organized as follicles in female (Figure 4A) and clusters in male (Figure 4D). Type I cells had subtypes of Type Ia and Type Ib. Type Ia cells were characterized by electron dense cytoplasm with rounded euchromatic nuclei, numerous secretory granules, Golgi apparatus, filamentous mitochondria and numerous parallel cisternae of rER in female (Figure 4B) and male (Figure 4E). Type Ib cells had electron lucent cytoplasm with rounded euchromatic nuclei, few secretory granules (S), Golgi apparatus, rER and filamentous mitochondria in female (Figure 4C) and male (Figure 4F). Type I cells had concentrated secretory granules near capillaries and rER on the opposite pole (Figure 4A) while type II cells have electron dense cytoplasm with oval nuclei of dense heterochromatin patches, rER and few secretory granules in female (Figure 4A) and male (Figure 4E).
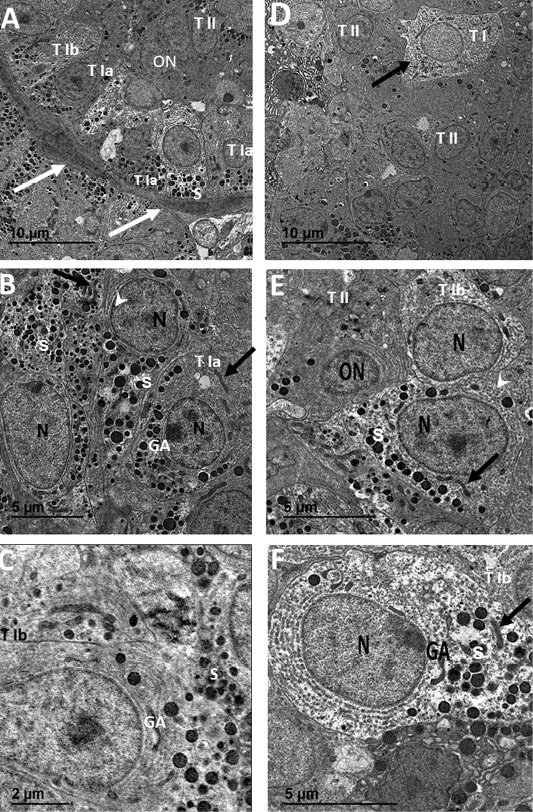
Figure 4: Transmission electron micrographs of SC from female catfish (A, B and C) showing septa housing numerous blood capillaries (white arrows) and surrounding follicular glandular cells of Type I with two subtypes; Type Ia (T1a), Type Ib (T1b) and Type II (TII), Type I cell with concentrated secretory granules (S) near capillaries and rER (arrowhead) on the opposite pole, Type II cells with small oval nuclei (ON) of dense heterochromatin patches and electron dense cytoplasm (white arrow) (A). Type Ia (TI a) has electron dense cytoplasm with rounded euchromatic nuclei (N), numerous secretory granules (S), Golgi apparatus (GA) and filamentous mitochondria (black arrows) (B). Type Ib (TI b) has electron lucent cytoplasm with rounded euchromatic nuclei (N), few secretory granules (S), Golgi apparatus (GA) and rER (black arrow head) (C). Transmission electron micrographs of SC from male catfish (D, E and F) showing glandular cells cluster of mainly Type II (TII) and sparse of Type I (T I) cells (A). Type II (TII) has electron dense cytoplasm with small oval nuclei (ON) of dense heterochromatin patches, rER and few secretory granules (S) (B). Type Ib (T Ib) cells have electron lucent cytoplasm with rounded euchromatic nuclei (N), few secretory granules (S), rER (white arrowhead), Golgi apparatus (GA) and filamentous mitochondria (black arrow) (B and C).
DISCUSSIONS
As far as we know, the previous studies did not address the comparison between Stannius corpuscles (SC) in male and female in African catfish. So, we focused on the histological and electron microscopical structures of SC gland in the both sexes. In the present investigation we revealed that the catfish SC are paired, white colored bodies. This result is in line with the SC in Notopterus notopterus fish (Bedjargi and Kulkarni, 2014a) and Rastrelliger kanagurta fish. On the other hand, Subhedar and Rao, 1976 found four bodies of SC in the catfish Heteroprenstes fossilis. Also, the Oncorhynclus tshawylscha and Oncorhynchus kisutch has five to six SC (Nadkarni and Gorbman, 1966). Interestingly, the SC of the present investigation was observed in the posterior region of the trunk kidneys. However, previous studies reported that SC could be embedded in the dorsal kidney mesonephros of Oncorhynchus fish (Nadkarni and Gorbman, 1966) and in the middle of mesonephros of salmonid fish and Atherinopsis californiensis (Krishnamurthy and Bern, 1969).
Histologically, the SC that we report here in the present study is surrounded by a thin CT. capsule that send septa to divide the gland into incomplete lobules. Septa were housing numerous blood capillaries in female catfish. The same observations are reported in killifish (Fundulus heteroclitus) (Cohen et al., 1975). We suggest that such well-developed vasculature as indication of the physiological function and activity of the gland as is mentioned in (Chester et al., 1969). Our observation revealed that the parenchyma of SC consisted of two types (I and II) of cells with different staining intensity (intensely and weakly stained cells) and arranged in follicles, clusters or cords. On the other hand, cells show large nuclei with granular cytoplasmic contents in Mystus tengara catfish (Chatterjee and Bhattacharjee, 2015). Interestingly, we revealed sexual dimorphism in the proportion of both cells. The predominant cell type in the female was the weakly stained cells while the vice versa in male, which might be, we suggest that correlated with the reproductive variance among both sexes.
Ultrastructurally, our study revealed the presence of two types of cells (type I and II). The prevalent type was type I (electron dense and electron lucent) in female that show rounded shape with round euchromatic nuclei and their cytoplasm showed the characteristic features of the active secreting cells including well developed Golgi complex, rER, filamentous mitochondria and secretory granules. These results are in accordance with the SC of two teleost species (trout and flounder) (Wendelaar Bonga et al., 1989), Oreochromis niloticus and Epinephilus tuvina (Abu Zinadah et al., 2006), and Notopterus notopterus (Chakrabarti and Banerjee, 2016). On the other hand, three types of cells were reported in the SC gland of Notopterus notopterus (Bedjargi et al., 2014a; Bedjargi et al., 2014b; Bedjargi and Kulkarni, 2014c) and Clarius batrachus (Ahmad et al., 2004).
Similar to that reported in Heteropneutes fossils, Notopterus notopterus and Rastrelliger kanagurta (Ahmad et al., 2004; Bedjargi and Kulkarni, 2014c; Bedjargi et al., 2014b), our results revealed that the cytoplasm of type II contain few filamentous mitochondria, strands of rER and with few or absence of secretory granules. Our results suggest that type II cells could be precursor for type I cells. However, further investigations are required to examine the structure of SC in both spawning and non-spawning seasons.
CONCLUSION
Our data revealed two types of cells forming the parenchyma of SC; weakly and intensely eosinophilic cells. Furthermore, we revealed sexual differences in the proportion of both cells. Ultrastructurally, the predominant cells showed more granules in their cytoplasm and could be considered as the cells that are responsible for hormone synthesis and secretion.
CONFLICT OF INTEREST
This manuscript has not been published or presented elsewhere in part or in entirety, and not under consideration by another journal. All the authors declare no competing financial interests.
Authors contribution
MK designed and performed the experiments, collected the data and wrote the manuscript. YE Conceived and designed the experiments, verified the analytical methods, edited the manuscript. HS designed the experiments, performed data interpretation, contributed discussion. MB designed the experiments and reviewed the manuscript.
REFERENCES






