Advances in Animal and Veterinary Sciences
Short Communication
Impact of Cepoka Eggplant Extract (Solanum torvum S.) and Kapok Seed (Ceiba pentandra G.) on Expression of p53 Protein and the Number of Leydig Cells in Rats
Viski Fitri Hendrawan*, Liza Sadda Cakrawati, Aulanni’am Aulanni’am, Desi Wulansari, Yudit Oktanella, Galuh Chandra Agustina
Faculty of Veterinary Medicine Brawijaya University, Puncak Dieng Eksklusif, Kalisongo, Dau Sub-districts, Malang Districts 65151.
Abstract | There is an increase population growth in Indonesia. Therefore, it is necessary to control this through the use of antifertility agents. Both cepoka eggplant (Solanum torvum S.) and Kapok seeds (Ceiba pentandra G.) have a role as antifertility agents as contains solasodine and gossypol compounds respectively. This study aims to determine the difference between the effect of cepoka eggplant extract and Kapok seed as male antifertility agents on the expression of p53 protein in the seminiferous tubular tissue and the number of Leydig cells in rats. A total of 18 male Wistar rats, 2.5-3 months old and 190-320 g weight were randomly divided into 3 groups with 6 replicats, as follows: the negative control group (K-) without treatment, group 1 (G1) given 1 g/kg Cepoka eggplant extract, and group 2 (G2) admistered 0.1 g/kg Kapok seed extract. The results showed that the administration of 0.1 g/kg Kapok seed extract (Ceiba pentandra G.) significantly increased the expression of p53 protein and reduced the number of Leydig cells compared with the administration of 1 g/kg Cepoka eggplant extract (Solanum torvum S.). It was concluded that Kapok seed extract can be used as antifertility agent as increase the expressions of p53 protein and reduce the number of Leydig cells.
Keywords | Cepoka Eggplant, Kapok Seed, Antifertility Agent, Protein p53, Leydig Cells
Received | May 28, 2019; Accepted | June 29, 2019; Published | August 24, 2019
*Correspondence | Viski Fitri Hendrawan, Faculty of Veterinary Medicine Brawijaya University, Puncak Dieng Eksklusif, Kalisongo, Dau Sub-districts, Malang Districts 65151; Email: [email protected]
Citation | Hendrawan VF, Cakrawati LS, Aulanniam A, Wulansari D, Oktanella Y, Agustina GC (2019). Impact of cepoka eggplant extract (solanum torvum s.) And kapok seed (ceiba pentandra g.) On expression of p53 protein and the number of leydig cells in rats. Adv. Anim. Vet. Sci. 7(9): 732-737.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.9.732.737
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Hendrawan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
In Indonesia, the high human population growth is a major demographic problem. The government efforts to solve this problem was via family planning program. Family planning program controls population growth through the use of contraceptives drugs or devices. Which is generally defined as a way to prevent pregnancy (Laili, 2017). This family planning program is not optimally functioning due to the low participation from the male as there is no method that fully safe and convenient for male. Thus, to raise male participation in family planning program needs a safe method which can be achieved through the natural antifertility drugs for male (Aulanni’am et al, 2007).
From veterinary medicine perspective, there are still many pets, such as dogs and cats, that live wildly and poorly maintained in our surroundings. These animals will continue to breed without any restriction from human that cause an overgrowth which result in that they will be poorly maintained or died. In addition to, there is negative impact on the animal health itself including zoonotic disease transmission. Although, the reproductive control of male animals is still limited to castration method.
In Indonesia, the research and development of contraceptive drugs are continuously reviewed and rapidly evolved. Various bioactive compounds in plants, particularly steroid, alkaloid, isoflavonoid, triterpenoid, and xanthone compounds, have a fertility regulator effect either through triggering or inhibiting (antifertility) (Hidayanti and Nofianti, 2014). The examples of this antifertility agents are Cepoka eggplant (Solanum torvum S.) and Kapok seeds (Ceiba pentandra G.).
Cepoka eggplant (Solanum torvum S.) is widely used by Indonesian society. Cepoka eggplant contains sterol carpesterol, alkaloid, steroid, and tannin compounds. These compounds can be used as antifertility agent. One of these alkaloids compounds is solasodine, as much as 0.84% (Hidayanti and Nofianti, 2014).
Kapok (Ceiba pentandra G.) trees grow in the beachside, 700 meters above sea level. Kapok seed contains chemical compounds as alkaloid, saponin, flavonoid, tannin, triterpenoid, and gossypol (Wirastuti et al, 2018). Gossypol in Kapok seed is used as an antifertility agent. Gossypol is a yellow phenolic aldehyde compound that has a toxic effect (Xin He et al, 2017).
This study was conducted to investigate the difference between the effect of Cepoka eggplant (Solanum torvum S.) and Kapok seed (Ceiba pentandra G.) extracts administration as a male antifertility agent on expression of p53 protein in seminiferous tubules and the number of Leydig cell in the Wistar rats (Rattus norvegicus).
MATERIALS AND METHODS
Experimental Design
A total of 18 male Wistar rats (Rattus norvegicus), 2.5-3 months old and 190-320 g weight were randomly divided into 3 groups (6, each) with 6 replicates (2, each), as follows: the negative control group (K-) without treatment, group 1 (G1) given 1 g/kg cepoka eggplant extract (Susilo and Akbar, 2016), and group 2 (G2) admistered 0.1 g/kg kapok seed extract (Gadelha et al., 2014). Rats were acclimatized for 1 week to environmental condition with ad libitum access to feed and water. The Cepoka eggplant and Kapok seed extract were administered for 10 days orally using gastric sonde. The usage of these experimental animals had been certified by Research Ethics Commission Brawijaya University number 967-KEP-UB. The rats were maintained in the Embryology Laboratory, Faculty of Veterinary Medicine, Airlangga University, Surabaya.
Cepoka Eggplant and Kapok Seed Extract Preparation
A 5 kg Cepoka eggplant fresh fruits were utilized. The extraction method was done by maceration using 70% ethanol as solvent. About 10,000 ml solvent were used for 3 hours evaporation time to form 2,000 ml extract. Also, 3 kg Kapok seed were used following the same method of extraction while 8000 ml solvent were used for 2 hours evaporation to form 700 ml extract (Nugroho, 2017).
Tissue Sampling
The testicle sample were obtained and stored in 10% formalin buffer. Fixation is done by inserting the organ into a 10% formalin buffer. The 10% formalin buffer contains 100 mL of formaldehyde 40% H.CHO, 4 grams of sodium monobasic phosphate NaH2PO4H2O, 6.5 grams of sodium phosphate dibasic Na2HPO4 and 900 mL of distilled water. After fixation, tissue samples need to be properly trimmed to reach the adequate size and orientation of the tissue (Slaoui and Fiette, 2010). Then, dehydration is done using a multilevel alcohol solution starting at 70%, 80%, 95% alcohol, absolute alcohol solutions I, II, and III for 1.5 hours. To do clearing, the tissue is transferred from absolute alcohol III to the purification solution, namely xylol I for 1 hour, xylol II and xylol III for 1.5 hours. Once tissue samples are infiltrated by paraffin, they are removed from the cassettes and carefully positioned inside metal base mold. Making block preparations using metal base molds (histo plate). Pour a small amount of paraffin liquid into the mold. Immediately enter the network using tweezers that have been heated (so that the paraffin is not frozen) and positioned in the mold. Liquid paraffin is then poured back to cover the entire mold. Preparation blocks are cut in thicknesses ranging from 5-7 micrometers. After that, the block pieces of the preparations that make up the paraffin tape are moved carefully using tweezers into a waterbath whose temperature is set 37-400C and left for a while until the paraffin tape expands. Paste paraffin tape on a coated glass object by inserting a glass object into the waterbath and moving it towards the paraffin tape. After the paraffin tape has firmly attached to the glass object, save the glass object containing paraffin pieces and tissue until it’s time to be colored. The coloring used is HE staining (Hematoxilin-Eosin).
Immunohistochemistry Preparation
The tissues were processed using an automatic tissue processor for ±18.5 hours. The tissue processing steps include fixating, dehydrating, clearing, embedding, paraffin infiltrating, blocking, mounting and staining.
Paraffin blocks containing seminiferous tubular tissues were cut into 3-4 μm thick and put on the poly-L-Lysine coated glass objects, then dried in the hotplate for ± 30 minutes. The poly-L-Lysine slides were then incubated overnight at 450C. The slides were deparaffinized using xylol I, xylol II, and xylol III and dehydrated with 96%, 90%, 80%, 70% absolute alcohol. Then, soaked in 3% hydrogen peroxide (H2O2 Block) for 5-10 minutes and rinsed with distilled water and PBS, 2 times for 5 minutes each.
After that, 0.025% Trypsin was conducted in the incubator at 370C for 15 minutes. The slides were rinsed again with Phosphat Buffer Saline (PBS), 2 times for 5 minutes each. Then, Ultra V Block was conducted for 5 minutes. The slides were rinsed with PBS and anti p53 monoclonal antibody. The anti p53 monoclonal antibody (5% diluted) was dripped on the slides and the slides were stored for 1 hour or overnight in the refrigerator at 80C. Then, rinsed again with PBS, 2 times for 5 minutes each. The slides were incubated with biotinylated link (yellow) antibody drops for 30 minutes, and then rinsed with PBS, 2 times for 5 minutes each. Followed by, streptavidin-peroxidase (red) was dripped on the slides for 30 minutes and the slides were rinsed with PBS, 2 times for 5 minutes each. Then, Diaminobenzidine (DAB) chromogen (2% diluted) and DAB plus substrate were dripped on the slides for 6-10 minutes, and the slides were rinsed with PBS, 2 times for 5 minutes each. Then, washed by distilled water for 5 minutes and stained with hematoxylin/Mayer for 5-10 minutes. Then, washed with running water for 5 minutes, followed by ammonia water for 3 minutes, and then dipped in the distilled water for 5 minutes (Aulanni’am, 2004).
Finally, the slides were mounted using canal/canada balm before the slides were covered with glass decks. The slides were observed under the microscope to investigate p53 expression in all experimental animal groups. The observation was conducted using a binocular microscope (Olympus® BX51 microscope digital camera system) at 400 time magnification. Cells expressing p53 protein were stained brown in the cell cytoplasm, whereas cells that didn’t express p53 protein were stained purple in the cell membrane. The averages percentage area of p53 protein expression in seminiferous tubular tissue and the number of Leydig cells were calculatetd using Immunoratio software.
Statistical Analysis
The data obtained for p53 protein expression and the number of Leydig cells based on the average percentage area analysis using ImmunoRatio software were analysed using SPSS with one-way ANOVA test (P<0.05) and then the difference between means were analysed by Honest Significantly Difference test (Tukey test) using P=5%.
RESULTS AND DISCUSSION
The p53 Protein Expression
The p53 protein expression in negative control (K-) group had the lowest intensity of a brown colour (Figure 1A) compared with treatment groups, while the Cepoka eggplant extract (T1) group (Figure 1B), and Kapok seed extract (T2) group (Figure 1C), showed a higher brown colour intensity compared with the negative control group.
This increase in p53 protein expression in seminiferous tubular tissues could be attributed to solasodine in Cepoka eggplant (Solanum torvum S.) extract effect on GnRH action in releasing FSH and LH during spermatogenesis as decreased its release which can result in cells shrunk and hypofunction (Hidayati and Nofianti, 2014).
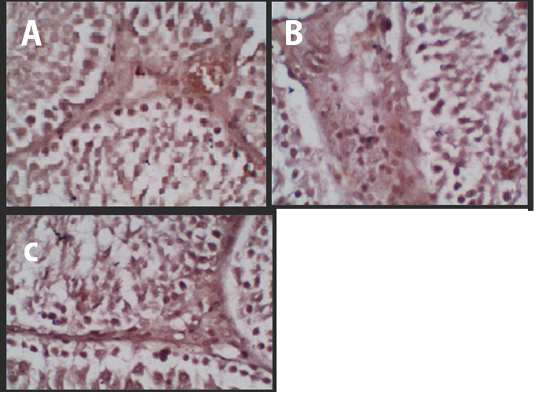
Figure 1 Immunohistochemistry of the p53 Protein Expression. (A) Negative Control, (B) Treatment 1 (T1) with Cepoka eggplant extract, (C) Treatment 2 (T2) with Kapok seed extract.
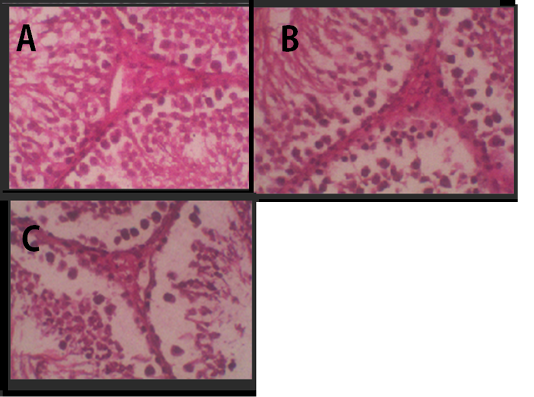
Figure 2: Histology of Leydig cells. (A) Negative Control, (B) Treatment 1 with Cepoka eggplant extract, (C) Treatment 2 with Kapok seed extract.
The disrupted FSH secretion can reduce Sertoli cells function to form Androgen Binding Protein (ABP), while the disrupted LH secretion can reduce Leydig cells function to form testosterone. Androgen Binding Protein (ABP) binds the testosterone to surround spermatogenic cells for maintaining the normal spermatogenesis activity.
Hidayati and Nofianti (2014), reported that flavonoid in Cepoka eggplant extract can inhibit the aromatase enzyme, an enzyme that catalyzes the conversion of androgen to estrogen, which results in increased androgen (testosterone) level. A high testosterone concentration sends negative feedback to hypothalamus so stop releasing FSH and LH. Furthermore, Sertoli and Leydig cells become hypofunction and didn’t produce ABP or testosterone.
In addition to ability of solasodine present in Cepoka eggplant (Solanum torvum S.) extract to compete the Follicle Stimulating Hormone (FSH) receptor thus disrupts the release of FSH from the hypothalamus which affects Sertoli cells which form Androgen Binding Protein. It can inhibit spermatogenic cell division so disrupt spermatogenesis (Kaspul, 2016). Also, can damage cell mitochondria, causing respiration process disruption and energy depletion for moving and maintaining cell life (Wahyuni, 2001).
However, T2 group using Kapok seed extract had a higher brown colour intensity compared with Cepoka eggplant extract (T1) group. That could be due to Kapok seed extract has a cytotoxic effect that widening membrane cell pores and can also damage the mitochondria that activate the p53 protein (Gadelha et al., 2014).
The results of the average percentage area measurement of p53 protein expression in each group were shown in Table 1.
Table 1: The different effects between Cepoka eggplant and Kapok seed extract in p53 protein expression in the seminiferous testicular tissue.
| Groups | The Average of p53 Protein Expression (%) ± SD | Increased p53 Protein Expression Compared with Negative control (%) |
| K- (Negative Control) |
72,9 ± 5,1a |
- |
| P1 (1 g/kg Cepoka eggplant extract) |
79,0 ± 10,0a |
8% |
| P2 (0.1 g/kg Kapok seed extract) |
96,9 ± 2,9b |
32% |
Note: (a,b) showed that there was significant p53 protein expression difference between the groups (α<0,05).
The Kapok seed (Ceiba pentandra G.) extract (T2) group showed a significantly (p<0.05) increased percentage area of p53 protein expression while Cepoka eggplant (Solanum torvum S.) extract (T1) group was not significantly different from the negative control group (p>0.05).
This could be attributed to the gossypol in Kapok seed extract which could damage the sperm mitochondria by activating the phospholipase and proteolytic enzyme in mitochondria membrane that inhibits the release and the use of ATP by sperm (Wirastuti et al, 2018). Gossypol binds connexin43 receptor in Sertoli cell and alter connexin43 receptor function. The connexin43 receptor facilitates communication between cells. The block of connexin43 receptor in the Sertoli cells delays Sertoli cells maturation (Gorji et al., 2015). Also, gossypol blocks the gap junction, the communication between sertoli cells and their surrounding cells. This gap junction is related to ion regulation in spermatogenesis. An important role of gap junction is to regulate cells growth and differentiation by controlling the small molecules traffic between adjacent cells (El-Sharaky et al, 2010).
Leydig Cell Number
Spermatogenesis is a complex process from germinal cell proliferation and spermatogonia maturation to form spermatozoa. A hormone that affects the spermatogenesis is testosterone, produced by Leydig cells. Leydig cell lies in interstitial tissue between the seminiferous tubules. Leydig cells are actually developed from cells that affect fibroblasts in the interstitial tissue and stimulated by LH. Luteinizing Hormone (LH) also stimulates the synthesis and secretion of the testosterone by Leydig cells (Foa, 2005).
Leydig cells number various according to its function. If testosterone is decreased, the spermatogenic cells production, including spermatogonia and primary spermatocyte cells, will also decrease in number (Foa, 2005).
In testicular histology, Leydig cells were tightly arranged in groups Figure 2. It was a large cell with vacuolated cytoplasm and many coarse chromatins in the nucleus that clearly seen. The cytoplasm was enriched with lipid. The agranular endoplasmic reticulum lied in the cell interstitial.
Table 2 showed that the average of Leydig cell number in Cepoka eggplant extract (T1) group and Kapok seed extract (T2) group were (24.8 and 23.5 respectively), which were significantly lower than that of the negative control. However, the decrease of the average number of Leydig cell in T2 group was higher.
Table 2: The average yield of leydig cells in rats in all treatments
| Treatment | Average Leydig Cells ± SD | Decrease Leydig Cells Toward Negative Control (%) |
| K1 (Negative Control) |
29,7±2,7a |
- |
| T1 (Treatment 1) |
24,8±1,9b |
16% |
| T2 (Treatment 2) |
23,5±1,8b |
20% |
The Leydig cell number decrease could be attributed to steroid alkaloid compound, particularly solasodine, that alter the hypothalamus function in releasing Gonadotropin Releasing Hormone (GnRH). The disrupted GnRH release will affect FSH and LH release, the hormones which maintain Leydig cell growth and testosterone secretion (Foa, 2005).
Furthermore, the steroid compound in Cepoka eggplant fruit (Solanum torvum S.) could reversibly inhibit spermatogenesis. It is because the high testosterone concentration send negative feedback to hypothalamus so stop releasing Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH) (Hidayati and Nofianti, 2014). Thus, LH secretion inhibition could disrupt LH signaling in Leydig cell for secreting testosterone.
Gossypol compound in Kapok seed (Ceiba pentandra G.) could disrupt spermatogenesis process due to testicular tissue degeneration including number of Leydig cells as it had a cytotoxic effect, thus, reduced sperma motility and its number, while, increase the number of abnormal sperm because the mitochondria in the sperm tail and germinal epithelium in seminiferous tubules were damaged (Gadelha et al, 2014; Wirastuti et al, 2018). The mitochondria function in the cell was to produce energy, in form of Adenosine Triphosphate (ATP) which used for cell homeostasis, regulation, division, and motility (Susmiarsih, 2010).
Testosterone hormone is highly required in the spermatogenesis process. Spermatogenesis process starts with the development of spermatogenic cell to form spermatozoa that ready to be released in the lumen of seminiferous tubules. The spermatozoa need testosterone to maintain its life in the epididymis. Besides, testosterone also plays a role in glucose uptake that will be metabolized in the mitochondria to produce ATP, the main energy source for spermatozoa to maintain its motility, activity, and life. Testosterone is needed to start the first meiosis process that forms secondary spermatocyte from primary spermatocyte. Furthermore, testosterone will maintain all development stages of spermatids. Testosterone hormone decrease lead to the release of abnormal spermatids from Sertoli cells to seminiferous tubules lumen that results in spermioogenesis failure (Prasetyorini et al, 2018). Therefore, the reduction of Leydig cell number can result in testosterone decreased which disrupt spermatogenesis. Finally, interrupted spermatogenesis process will lead to infertility.
CONCLUSION
It could be concluded that the administration of 0.1 g/kg body weight Kapok seed extract (Ceiba pentandra G.) increased the expression of p53 protein and reduced the number of leydig cells in the rats (Rattus norvegicus) compared with the administration of 1 g/kg Cepoka eggplant extract (Solanum torvum S.). So, Kapok seed extract can be developed as male antifertility agent.
COnflict of interest
The authors declare that they have not conflict of interest.
AcknowledgmentS
The researcher would like to thank the Embryology Laboratory Faculty of Veterinary Medicine Airlangga University Surabaya, Anatomical Pathology Laboratory Faculty of Veterinary Medicine Airlangga University Surabaya, and the Joint Research Laboratory Faculty of Veterinary Medicine Brawijaya University Malang for the permission during this study.
Authors Contribution
Viski Fitri Hendrawan: conducted the study, collected the data, and drafted the manuscript. Liza Sadda Cakrawati, Aulanni’am Aulanni’am, Desi Wulansari, Yudit Oktanella and Galuh Chandra Agustina participated in draft and improved the manuscript.
REFERENCEs






