Advances in Animal and Veterinary Sciences
Research Article
The Cytogenetic Effects of Levofloxacin in Male Rats
Wael. F. Al-Soufi1*, Falah Muosa Kadhim Al-Rekabi2
1Department of Medical and Biological Supervision, Veterinary Directorate, Iraq; 2College of Veterinary Medicine, University of Baghdad, Iraq.
Abstract | This study was conducted to evaluate the cytogenetic effects of levofloxacin in male rats. It was carried out on fifty four of the albino male rats, they were divided equally into three main groups and orally dosed levofloxacin and assigned as, the first group was dosed with the therapeutic dose 7.5mg/kg/bw, the second group was dosed with the double therapeutic dose 15mg/kg/bw, the third group was dosed with distilled water and became a control group. The three groups were divided into three subgroups equally according to dosing period of 2 weeks, 4 weeks, 1 week after discontinuation of dosing. Collection of blood and scarification of group animals were done at the end of each period. The results showed, that there were no significant P>0.05 differences in both the micronuclei assay and in chromosomal aberration among the experimental groups, while the results of both nuclear division index and mitotic index showed, a significant P<0.05 decrease in their values in both dosed drug groups after the fourth week of the dosage compared to other treated periods and control group. The results of the comet assay showed, varying of results with significant P<0.05 decreases in both treated groups compared to the control group.We concluded that levofloxacin is a cytotoxic but not genotoxic agent.
Keywords | Cytogenetic, Effect, Levofloxacin, Male Rat.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | September 06, 2018; Accepted | November 11, 2018; Published | December 29, 2018
*Correspondence | Wael F Al-Soufi, Department of Medical and Biological Supervision, Veterinary Directorate, Iraq; Email: [email protected]
Citation | Al-Soufi WF, Al-Rekabi FMK (2019). The cytogenetic effects of levofloxacin in male rats. Adv. Anim. Vet. Sci. 7(3): 138-150.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.3.138.150
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Al-Soufi and Al-Rekabi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The main active substances of quinolones and fluroquinlones are cinoxacin, ciprofloxacin, enoxacin, flumequin levofloxacin, lomefloxacin, moxifloxacin, nalidixic acid, norfloxacin, ofloxacin, pefloxacin, pipemidic acid, prulifloxacin, and rufloxacin. broad spectrum antibiotics, which are widely prescribed and are important for treating serious, life threatening bacterial infections (European Medicine Agency, 2018; Blessed et al., 2018).
Fluroquinolones are now being used for treatment of various bacterial diseases in animals. An emergence of bacterial resistance against this class of drugs needs great efforts to evaluate the newer fluroquinolones for therapy in human and veterinary medicine (Patel et al., 2012a).
Levofloxacin is a third-generation fluoroquinolone that possesses activity against most aerobic Gram-positive and Gram-negative organisms and demonstrates moderate activity against anaerobes, as well as atypical pathogens such as Mycoplasma and Chlamydia (Aboubakr and Soliman, 2014). The inhibition of DNA gyrase, (enzyme required for DNA replication, transcription, repair and recombination) is the specific mechanism of action of levofloxacin and other fluoroquinolones antibacterials (Bano et al., 2014).
Levofloxacin is well distributed toward target body tissues, so it uses in treatment of urinary tract infection (complicated and uncomplicated) and community-acquired pneumonia, including multidrug resistant strains of several bacteria (Levaquin, 2018).
In veterinary medicine Levofloxacin is indicated to use in poultry (chicken, duck, goose) and Pigs bacterial infection by E. coli, staphylococcus infection, ovarian inflammation (Tubo-ovarian abscess), green suppuration bacillus, yellow and white dysentery, pericarditis, enteritis and infection caused by cholera etc. (Kyuchukova et al., 2013; Patel et al., 2012b).
Mahendra et al., 2011)Sheep mastitis, Pneumonia, Acute bacterial sinusitis, Acute and Chronic bronchitis, Skin infections, Urinary tract infections, Acute pyelonephritis and repeat breeding syndrome in cow (Kumar et al., 2014; Patel et al., 2012c).
Arthropathy, have been shown in immature dogs (4–5 months old) after oral doses of levofloxacin at 10mg/kg/day for seven days and after fourteen days after intravenously doses at 4mg/kg/day (Vetinfo, 2012), in juvenile rats after oral doses of levofloxacin 30mg/kg/day for seven days and intravenous doses of 60 mg/kg/day for four weeks (Levaquin, 2018).
The antibacterial effect of FQs is due to their inhibition of the bacterial topoisomerase type II enzymes, such as bacterial gyrase. Type II topoisomerases are essential nuclear enzymes found in prokaryotic and eukaryotic cells that regulate the topological state of DNA during replication, transcription and repair. During the topoisomerase II cycle, the enzyme covalently binds to DNA and produces temporary double-strand breaks, thus creating atransient gate (cleavage complex) through which another DNAduplex can pass. After strand passage the break is ligated and the DNA structure is restored. Numerous compounds are known to disrupt the DNA breakage–reunion cycle of mammalian topoisomerase II. This disruption during DNA transcription or replication can result in DNA strand breaks being exposed and this may lead to clastogenicity and/or cytotoxicity if the exposed DNA strand breaks are not repaired (Lynch et al., 2003).
Due to levofloxacin role on DNA transcription in human and animals, this study conducted to investigate the cytogenetic effects in male rats.
Materials and Methods
Experimental Animal Housing
Fifty four healthy adult albino male rats were obtained from the National Center for Drug Control and Research (NCDCR) / Ministry of Health. Their ages ranged between 6 – 8 months, and their weight ranged 500 – 600g. They were kept under suitable environmental conditions of 20-25˚C. Food and water were offered. Care was taken to avoid any unnecessary stress. The animals were kept for at least two weeks for adaptation before starting the experiment. Experiments of this study were conducted in the animal house and laboratories of the Department of the Medical and Biological supervision / Veterinary Directorate.
Experimental Design and Animal Grouping
It was conducted under the approval of scientific committee of department of Physiology, Biochemistry and Pharmacology, College of Veterinary Medicine, University of Baghdad and takes inconsideration the standard ethic of animal welfare.
Fifty four male rats divided randomly and equally into three groups treated for 4 weeks as, with levofloxacin through gastric gavage and assigned as, group 1(G1) dosed therapeutic dose 7.5mg/kg/bw, group 2 (G2) dosed 15mg/kg/bw, while third group dosed distilled water and considered control group, furthermore each group subdivided into three subgroups equally according the period of treated to levofloxacin which were included 2, 4 weeks of dosing and 1 week after discontinuing levofloxacin administration.
Blood Sampling
Five ml of blood was collected in heparinized tubes by heart puncture technique after rats got general anesthesia through intraperitonial (IP) injection with (Ketamine 100mg/kg/b.w and xylazine 13mg/kg/b.w).
Cytogenetic Assessment
They were included micronucleus assay according to method of (Fenech, 2000) briefly by adding cytochalasine B to blood samples for obtaining nuclear division index (NDI) and micronucleus frequency (MNi). These parameters were calculated by using the following formula:
NDI= [1(M1) +2(M2) +3(M3) +4(M4)]/N
MNi= [1(MN1) +2(MN2) +3(MN3) +4(MN4)/N]
NDI=Nuclear division index.
M 1, 2, 3, 4=Number of nucleate cells
MN 1, 2, 3, 4=Number of micronucleus in cells.
N= Total number of cells.
Chromosomal aberrations (CA) and mitotic index(MI) were investigated in bone morrow stem cells according to the method of (Watt and Stephen, 1986; Block, 1999)which mitotic index was calculated by following formula:
M.I. %=No. of dividing cells in metaphase / (Total No. of dividing cells +No. of non- dividing cells (1000) cells) X 100 (Becker, 1986).
While, chromosomal aberrations were observed in each 100 metaphase cells (Lamberti et al., 1983).
Comet assay was performed on bone marrow according to the method of (Olive et al., 1990; DeBoeck et al., 2000).
Fifty randomly selected cells were counted per sample to quantify the comet cell. Scored was calculated from the ratio of length to width (L/W) comet to determine the Comet Index (CI). Scored range from 1.2 to 2 considered low DNA damage (LD), from 2.1 to 3 medium DNA damage (MD), and up to 3 high DNA damage (HD) (Collins et al., 2003; Al-Jewari, 2010).
The quantification of comet scoring by using image analysis software (Figure 1), the analysis software calculate different parameters for each comet, 4 parameters were evaluated to indicate DNA migration, tail length (distance from the head center to the end of the tail), tail moment (product of tail DNA/total DNA by the tail center of gravity), DNA% in tail (100X Tail DNA Intensity/Cell DNA Intensity) and olive moment (product of the tail length and the fraction of total DNA in the tail) (Gontijio et al., 2001; Azqueta et al., 2009).
Statistical Analysis
Analysis of variance and SAS (Statistical Analysis System - version 9.13). Two way ANOVA and Least significant differences (LSD) post hoc test were performed to assess significant differences among means. P<0.05 was considered statistically significant (SAS, 2010).
Results
Nucleus Division Index (NDI)
The results of NDI showed, significant p<0.05 decreases within treated groups (G1 and G2) after 4 weeks of levofloxacin dosing (7.5 and 15) mg/kg/b.w respectively, in comparison with that after 2 weeks dosing, but the NDI values in both treated groups (G1 and G2) regain normal value since the animals of these groups revealed no significant P>0.05 changes after 1 week of levofloxacin withdrawal when compared to the after 2 weeks values and control group. No significant P>0.05 changes showed, within control group along the experimental period. While the results between groups showed no significant P>0.05 changes along all the experimental periods. (Table 1) (Figure 2).
Table 1: Nucleus Division Index Values
| Groups | 2 Weeks | 4 Weeks | Withdrawal |
|
Group 1(G1) Levofloxacin 7.5mg/kg bw No=6 |
2.13 ± 0.06
A a |
1.80 ± 0.10
B a |
2.01 ± 0.01
AB a |
|
Group 2(G2) Levofloxacin 15mg/kg bw No=6 |
2.14 ± 0.14
A a |
1.85 ± 0.07
B a |
2.03 ±0.02
AB a |
|
Control (C) D.W, No=6 |
1.92 ± 0.09
A a |
1.97 ± 0.06
A a |
1.91 ± 0.06
A a |
| LSD | 0.2386 | ||
Values = mean ± SE
Means with different capital letters denote significant differences (P<0.05) within group.
Means with different small letters denote significant differences (P<0.05) between groups.
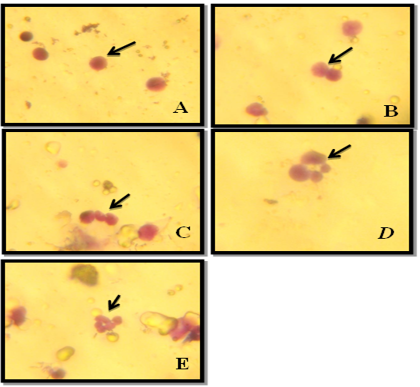
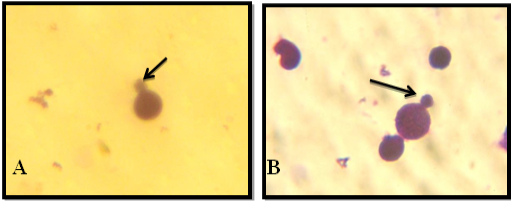
Figure 2: NDI Under Light Microscope (100x), A: Mononucleated, B: Binucleated, C: Trinucleated, D: Tetranucleated and E: pentanucleated
Micronucleus MN
The results of MNi showed, no significant p>0.05 differences within all experimental groups (G1, G2 and C) and between these groups too (Table 2) (Figure 3).
Table 2: Micronucleus Frequency Values
| Groups | 2 Weeks | 4 Weeks | Withdrawal |
|
Group 1(G1) Levofloxacin 7.5mg/kg bw No=6 |
2.11 ± 0.0
A a |
2.08 ± 0.24
A a |
2.14 ± 0.01
A a |
|
Group 2(G2) Levofloxacin 15mg/kg bw No=6 |
2.26 ± 0.07
A a |
2.24 ± 0.06
A a |
2.10 ± 0.05
A a |
|
Control (C) D.W, No=6 |
2.00 ± 0.05
A a |
2.02 ± 0.06
A a |
1.89 ± 0.03
A a |
| LSD | 0.2797 | ||
Values = mean ± SE
Means with different capital letters denote significant differences (P<0.05) within group.
Means with different small letters denote significant differences (P<0.05) between groups.
Table 3: Mitotic Index Frequency Values
| Groups | 2 Weeks | 4 Weeks | Withdrawal |
|
Group 1(G1) Levofloxacin 7.5mg/kg bw No=6 |
16.37 ± 3.52
A a |
9.75 ± 3.85
B b |
12.75 ± 1.19
AB a |
|
Group 2(G2) Levofloxacin 15mg/kg bw No=6 |
15.22 ± 0.31
A a |
8.50 ± 1.36
B b |
10.10 ± 2.73
AB a |
|
Control (C) D.W, No=6 |
12.00 ± 1.58
A a |
16.75 ± 2.28
A a |
14.25 ± 2.17
A a |
| LSD | 6.8799 | ||
Values = mean ± SE
Means with different capital letters denote significant differences (P<0.05) within group.
Means with different small letters denote significant differences (P<0.05) between groups.
Mitotic Index
The results of MI showed, significant p<0.05 decreases within treated groups (G1 an G2) after 4 weeks of levofloxacin dosing (7.5 and 15) mg/kg/b.w respectively, in comparison with that after 2 weeks dosing, but the MI values in both treated groups (G1 and G2) regain normal value since the animals of these groups revealed no significant P>0.05 changes after 1 week of levofloxacin withdrawal when compared to the after 2 weeks values and control group. No significant P>0.05 changes showed, within control group along the experimental periods. While the results between groups showed, there were significant P<0.05 decreases after 4 weeks of treatment in treated groups (G1 and G2) in comparison with control group, but there were no significant P>0.05 changes after 2 weeks of treatment and 1 week of levofloxacin withdrawal along the experimental periods (Table 3).
Chromosomal Aberration
There were several chromosomal aberrations observed in all experimental groups along the study periods which included dicentric, acentric and ring chromosomes as well as chromatid breaks and deletion in several chromosomes in different treated groups in variable numbers (Figure 4). In details, the results of total chromosomal aberrations showed, no significant P>0.05 differences within all the experimental periods of control group C, whereas there were significant P<0.05 decrease within G1 (levofloxacin 7.5 mg/kg/bw) and G2 (15mg/kg bw) after 4 weeks of levofloxacin dosing and withdrawal, when compared with the 2 weeks of levofloxacin dosing, but there were no significant P>0.05 differences after 4 weeks of levofloxacin dosing compared with withdrawal period of levofloxacin dosing within same groups (G1 and G2). While the results between experimental groups (G1, G2 and C), showed, no significant P>0.05 differences after 4 weeks of levofloxacin dosing and after withdrawal levofloxacin dosing. only after 2 weeks of treatment there were variable changes in total chromosomal aberration between all experimental groups, whereas G1 showed, significant P<0.05 decrease while G2 showed, significant P<0.05 increase when compared to control group (Table 4) (Figure 4).
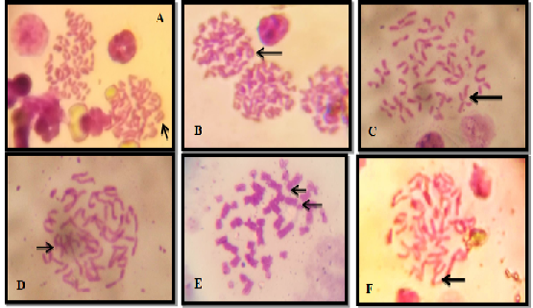
Figure 4: Chromosomal Aberration Under Microscope (100x), A. Dicentric Chromosome. B. Ring Chromosome. C. Chromatid Break D. Acentric Chromosome. E. Acentric Break Chromatide and F. Deletion Chromosome
Comet Assay Results
Microscopic Examination: The results of comet assay are summarized in (Table 5). There were no significant P>0.05 differences in comet assay outcomes between all experimental groups (G1,G2 and Control) after 2 weeks of treatment, except group 2 (Levofloxacin 15mg/kg/bw) showed, significant P<0.05 decrease in high level comet in comparison to both G1 and Control groups.
Table 4: Chromosomal Aberrations / Total Values
| Groups | 2 Weeks | 4 Weeks | Withdrawal |
|
Group 1(G1) Levofloxacin 7.5mg/kg bw No=6 |
0.875 ± 0.07
A b |
0.250 ± 0.00
B a |
0.562 ± 0.06
B a |
|
Group 2(G2) Levofloxacin 15mg/kg bw No=6 |
1.187 ± 0.06
A a |
0.437 ± 0.18
B a |
0.375 ± 0.12
B a |
|
Control (C) D.W, No=6 |
0.375 ± 0.07
A c |
0.500 ± 0.14
A a |
0.562 ± 0.11
A a |
| LSD | 0.3122 | ||
Values = mean ± SE
Means with different capital letters denote significant differences (P<0.05) within group.
Means with different small letters denote significant differences (P<0.05) between groups.
After 4 weeks of treatment, the experimental groups showed, variable outcomes of comet levels, which could be summarized as following, G1 (7.5mg/kg/bw) showed significant p<0.05 increase in no comet level in comparison to both G2 and control groups. Also, G1 and G2 showed significant P<0.05 decrease in high level comet when compared to control group, so both treated groups with levofloxacin, showed no significant P>0.05 changes in low and moderate comet level in comparison to the control group.
After one week of levofloxacin withdrawal, only treated group G1showed, significant P<0.05 decrease in high comet levels in comparison to control group, while there were no significant P>0.05 differences in no, low and moderate comet levels between all experimental groups.
The results of comet levels within groups, are explaining in (Table 5) also, and could be summarized as following, G1 treated with levofloxacin (7.5mg/kg/bw) showed, significant P<0.05 decrease in high comet level after 4 weeks and one week of levofloxacin withdrawal, and low comet level after one week of levofloxacin withdrawal in comparison with comet level changes after 2 weeks of treatment.G2 treated with levofloxacin (15mg/kg/bw) showed, significant P<0.05 decrease in no comet level after 4 weeks of tr-
Table 5: Comet Assay Analysis Values
| Period | Comet Types |
Group 1(G1) Levofloxacin 7.5mg/kg BW No=6 |
Group 2(G2) Levofloxacin 15mg/kg BW No=6 |
Control (C) D.W No=6 |
|
2 weeks |
No | A 38.12±2.86 a | A 42.95±5.81 a |
A 39.17±1.74 a |
| Low | A 30.77±2.92 a | A 32.42±1.88 a | A 27.72±2.69a | |
| Mod. | A 19.17±0.86 a | A 17.55±2.07 a |
A 20.32±1.19 a |
|
| High | A 16.77±1.46 a |
B 7.87±2.15 cb |
A 16.62±1.18 c |
|
|
4 weeks |
No | A 41.25±2.81 a | B 34.52±1.82 b |
B 36.07±1.06 a |
| Low | A 31.17±2.32 a | A 28.52±0.83 a |
A 26.05±0.62 a |
|
| Mod. | A 17.95±1.47 a | A 18.60±1.47 a |
A 18.67±0.28 a |
|
| High | B 7.05±1.09 b |
B 8.00±1.48 b |
A 17.17±0.57a | |
|
Withdrawal |
No | A 40.45±1.32 a | A 37.55±1.33 b | A 35.02±0.29 a |
| Low | A 24.80±0.94 b | A 23.42±2.04 b | A 26.00±1.24 a | |
| Mod. | A 19.42±0.67 a | A 22.60±1.18 a | A 21.35±0.39 a | |
| High | B 9.50±0.39 b | AB 12.17±1.44 a | A 16.32±0.56 a | |
|
LSD |
|
5.1387 |
|
|
Values Means = ± SE
Means with different small letter in the same column significantly different (P< 0.05); Means with different capital letter in the same row significantly different (P< 0.05)
eatment and one week of levofloxacin withdrawal in comparison to no comet level after 2 weeks of treatment, and also showed significant P<0.05 decrease in low comet level after one week of levofloxacin withdrawal only in comparison to both 2 and 4 weeks of treatment. While moderate comet level showed no significant P>0.05 changes along all the experimental periods, but there was significant P<0.05 increase in high comet level after one week of levofloxacin withdrawal in comparison to the same comet degree of comet level after 2 and 4 weeks of treatment with levofloxacin (Table 5) (Figure 5 & 6).
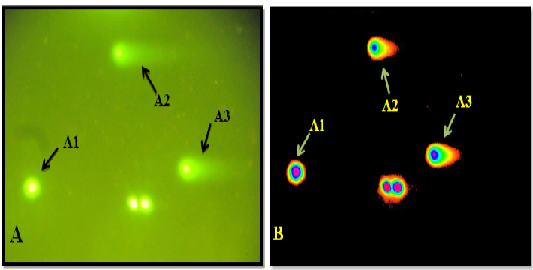
Figure 5: A: Under light microscope A1. No comet A2. Low comet A3. Moderate comet. B: Microscopic picture analyzed by Tri Tek Comet ScoreTM Freeware v1.5.
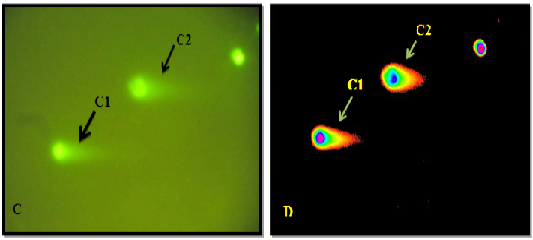
Figure 6: C: Under light microscope C1 & C2 Shows High Comet. D: Microscopic picture analyzed by Tri Tek Comet ScoreTM Freeware v1.5.
Comet Scoring
Tail length: The results of tail length showed no significant P>0.05 differences within all experimental groups (G1, G2 and C) along experimental periods, while the results between groups showed, no significant P>0.05 differences after 2 & 4 weeks. But after withdrawal of levofloxacin dosing there were no significant P>0.05 differences between G1 (7.5 mg/kg/bw) when compared with control group, while G2 (15mg/kg/bw) showed significant P<0.05 decrease when compared with G1 and no significant P>0.05 changes in comparison with control group (Table 6).
%DNA in Tail: The results of %DNA in Tail, showed no significant P>0.05 differences between all experimental groups while within experimental groups G1 (7.5mg/kg /bw) and C along experimental periods showed no significant P>0.05 differences but only group 2 (15mg/kg /bw) exhibited no significant P>0.05 differences after 2 and 4 weeks dosing of levofloxacin, whereas after one week of withdrawal of levofloxacin dosing there was significant P<0.05 decrease in comparison with 2 weeks and 4 weeks of levofloxacin dosing period (Table 7).
Table 6: Comet Scoring of Tail Length Values
| Groups | 2 weeks | 4 weeks | Withdrawal |
|
Group 1(G1) Levofloxacin 7.5mg/kg bw No=6 |
20.90 ± 9.48
A a |
8.50 ± 7.72
A a |
21.00 ± 8.23
A a |
|
Group 2(G2) Levofloxacin 15mg/kg bw No=6 |
10.60 ± 4.27
A a |
10.60 ± 4.84
A a |
4.60 ± 3.60
A b |
|
Control (C) D.W, No=6 |
7.50 ± 2.65
A a |
7.00 ± 2.20
A a |
8.00 ± 2.09
A ab |
| LSD | 15.969 | ||
Values = mean ± SE
Means with different capital letters denote significant differences (P<0.05) within group.
Means with different small letters denote significant differences (P<0.05) between groups.
Table 7: Comet Scoring / %DNA in Tail Values
| Groups | 2 weeks | 4 weeks | Withdrawal |
|
Group 1(G1) Levofloxacin 7.5mg/kg bw No=6 |
23.50 ± 9.96
A a |
9.38 ± 7.91
A a |
9.73 ± 5.24
A a |
|
Group 2(G2) Levofloxacin 15mg/kg bw No=6 |
14.49 ± 6.20
A a |
20.98 ± 7.68
A a |
1.24 ± 0.86
B a |
|
Control (C) D.W, No=6 |
18.08 ± 4.02
A a |
18.24 ± 4.03
A a |
18.06 ± 3.97
A a |
| LSD | 17.193 | ||
Values = mean ± SE
Means with different capital letters denote significant differences (P<0.05) within group.
Means with different small letters denote significant differences (P<0.05) between groups.
Tail Moment: The results of tail moment showed, no significant P>0.05 differences within all experimental groups (G1, G2 and C) along experimental periods, while the results between experimental groups showed, no significant P>0.05 differences after 4 weeks and withdrawal of levofloxacin dosing. But after 2 weeks of levofloxacin dosing there were no significant P>0.05 differences between G1 (7.5 mg/kg/bw) when compared with G2 group (15mg/kg/bw), but their values exhibited, significant P<0.05 increase when compared to the control group (Table 8).
Olive moment: The results of olive moment, showed no significant P>0.05 differences within all experimental groups (G1, G2 and C) along experimental periods, while the results between test groups showed, no significant P>0.05 differences after 4 weeks and withdrawal of levofloxacin dosing. But after 2 weeks of levofloxacin dosing only G1 which was treated by levofloxacin (7.5 mg/kg/bw) showed, significant P<0.05 increase in olive moment when compared with the control group, while G2 which was treated with levofloxacin (15 mg/kg/bw) didn’t do so (Table 9).
Table 8: Comet Scoring of Tail Moment Values
| Groups | 2 weeks | 4 weeks | Withdrawal |
|
Group 1(G1) Levofloxacin 7.5mg/kg bw No=6 |
14.79 ± 9.43
A a |
6.25 ± 6.23
A a |
5.73 ± 3.69
A a |
|
Group 2(G2) Levofloxacin 15mg/kg bw No=6 |
3.73 ± 1.67
A a |
5.34 ± 3.08
A a |
0.32 ± 0.31
A a |
|
Control (C) D.W, No=6 |
2.07 ± 0.89
A b |
2.17 ± 0.79
A a |
2.36 ± 0.86
A a |
| LSD | 11.716 | ||
Values = mean ± SE
Means with different capital letters denote significant differences (P<0.05) within group.
Means with different small letters denote significant differences (P<0.05) between groups.
Table 9: Comet Scoring of Olive Moment Values
| Groups | 2 weeks | 4 weeks | Withdrawal |
|
Group 1(G1) Levofloxacin 7.5mg/kg bw No=6 |
12.43 ± 6.52
A a |
4.62 ± 4.07
A a |
6.31 ± 3.54
A a |
|
Group 2(G2) Levofloxacin 15mg/kg bw No=6 |
5.18 ± 2.24
A ab |
5.93 ± 2.42
A a |
0.70 ± 0.60
A a |
|
Control (C) D.W, No=6 |
2.31 ± 0.57
A b |
2.08 ± 0.57
A a |
2.18 ± 0.58
A a |
| LSD | 8.5966 | ||
Values = mean ± SE
Means with different capital letters denote significant differences (P<0.05) within group.
Means with different small letters denote significant differences (P<0.05) between groups.
Discussion
The main objective of this study was to evaluate if the exposure of levofloxacin with therapeutic dose 7.5mg/kg/b.w and double therapeutic dose 15mg/kg/b.w, induced increases in the levels of cytogenetic damage. The study was carried out in parallel with an exposed and control rats groups. To evaluate the chromosome damage as measured by nuclear division index NDI, mitotic index MI, micronuclei MN and chromosomal aberration CA, peripheral lymphocytes have been used for detecting genotoxic effects, from rats’ blood to detect NDI and MN, these cells are in a non-proliferative stage (G0) and have a long half-life about 3 years (Pastor et al., 2001). Extracted bone marrow stem cells were used to detect MI, CA and level of DNA damage (Comet Assay).
Due to importance of determining the genotoxic and cytotoxic effects of the antibiotics as well as other various side effects, several studies has been conducted on the possible genotoxic potentials of fluoroquinolones including levofloxacin by using different in vivo and in vitro test systems (Brambilla, et al., 2012; Smart and Lynch, 2012; Peacock, 2014).
Itoh et al. (2002) examined the photochemical clastogenic potential of 12 antibacterial agents from quinolone group (sparfloxacin, clinafloxacin, gemifloxacin, lomefloxacin, sitafloxacin, grepafloxacin, fleroxacin, enoxacin, levofloxacin, moxifloxacin, trovafloxacin, and DK-507k (novel quinolone) in cultured Chinese Hamster Lung (CHL) cells by in vitro chromosomal aberration test. Following the light radiation, it was observed that antibacterial agents (except DK-507k) have caused to an increase in the frequency of cells containing structural aberrations. Researchers reported that photochemical and nonphotochemical clastogenic potentials of quionole antibacterial agents have decreased by the displacement of methoxy group at theC-8 position of quinole core.
Reus et al. (2012) have found that sporloxacin and lomefloxacin significiantly increased MN frequency in the skin of mouse by in vivo photomicronucleus test but the increase observed in treated cells with ciprofloxacin and levofloxacin was not at significant level, these results are consistent with our study, where there were no significant P>0.05 changes in MNi of levofloxacin treated groups.
Shimada et al. (1992) investigated the mutagenic effect of levofloxacin by using Ames test, HGPRT mutation test, SCE test in CHL cells, MN, CA, and SCE test in mouse bone marrow, in vivo-in vitro unscheduled DNA synthesis test (UDS) in rat primary hepatocytesa nd the dominant lethality test in BDF1 mice. Levofloxacin, induced CA and SCE frequency in a dose dependent manner in CHL cells, whereas no mutagenic effect was observed in other tests.
Zhu et al. (2012) have investigated the genotoxic effect of levofloxacin n-oxid 1mg/ml isolated from levoflaxacin by CA test in Chinese hamster lung CHL cells. Although the test substance caused a significant increase in the number of metaphases with structural aberration, the test impurity was not mutagenic in the test of mouse lymphoma assay (MLA), the outcomes of this study is inconsistent with the finding of our study, where there were no significant P>0.05 changes in MNi. The thought is may be due to the model of the test when Zhu et al. (2012) study performed in tissue culture in comparison without in vivo study, consequently the differences between them due to metabolic role in vivo model.
Zhu et al. (2013) found that decarboxylated levofloxacin isolated from levofloxacin did not show mutagenic activity in the Ames test, but significantly increased the number of cells containing structural aberration in CHL cells rather than structural aberrations.
Tan et al. (2012) found that levofloxacin significantly reduced cell viability and hyaluronan level, while increasing apoptosis and activecaspase-3 levels in rabbit fibroblast-like synoviocyte cells (FLS) and indicated that levofloxacin have cytotoxic effect on FLS cells.
Deng et al. (2011) observed that levofloxacin increased apoptosis in rabbit anteriorcruciate ligament cells (ACL) treated with levofloxacin but reduced the amount of extracellular matrix and they reported that levofloxacin have cytotoxic effect on these cells.
Kayraldiz et al. (2017) found that treatment with the different doses of levofloxacin for 24 and 48h did not affect the SCE frequency, but highest levofloxacin concentration (100μg/ml) caused a significant increase in the CA level. Also, treatment of 25, 50, and 100μg/ml levofloxacin significantly increased the MN level as compared the control group. There were no significant differences between the treated cells and control according to the proliferation index, mitotic index, and nuclear division index.
In this study, possible genotoxic effects of levofloxacin were investigated by using in vivo CA and in vitro MN tests. Treatment with different doses of levofloxacin, therapeutic dose 7.5mg/kg/b.w and double therapeutic dose 15mg/kg/b.w along 2 and 4 weeks, and after one week withdrawal of levofloxacin dosing, there was a significant increase in the CA levels after 2 weeks of levofloxacin dosing in both groups as compared to control group, but after 4 weeks dosing the CA values in both treated groups (G1 and G2) regain normal since the animals of these groups revealed no significant P>0.05 changes after 1 week of levofloxacin withdrawal in comparison with control group. CA results of our study are in consistent with the findings of (Itoh et al., 2002; Shimada et al., 1992; Zhu et al., 2013) from the CHL cells; (Tan et al., 2012; Deng et al., 2011) in rabbit ACL cell, and (Kayraldiz et al., 2017) in human peripheral lymphocytes. Similarly with (Reus et al., 2012; Shimada et al., 1992; Zhu et al., 2013), levofloxacin resulted with no significant increase in MN frequency in all treated animals groups as compared to control group.
Also, inpresent study, the MI, and NDI values of the treated animals were also determined to investigate the cytotoxic effect of levofloxacin. There were significant P<0.05 decreases observed in MI and NDI after 4 weeks of treated animals groups as compared to control group with continuous regain to normal values after 1 week of levofloxacin withdrawal in comparison with control group. The study reported cytotoxic effect of levofloxacin same as what they found on FLS and rabbit ACL cells (Tan et al., 2012; Deng et al., 2011), and in contrary to that found by Kayraldiz et al. (2017).
Albertini et al. (1995); Oliphant and Green, (2002) describes an evaluation of the predictive capacity of the in vivo rat peripheral lymphocytes using different concentrations of levofloxacin. The interaction of some FQ antibiotics with the mammalian topoisomerase II enzyme is responsible for their genotoxic potential in mammalian organisms.
The antibacterial effect of FQs is due to their inhibition of the bacterial topoisomerase type II enzymes, such as bacterial gyrase. Type II topoisomerases are essential nuclear enzymes found inprokaryotic and eukaryotic cells that regulate the topological state of DNA during replication, transcription and repair. During the topoisomerase II cycle, the enzyme covalently binds to DNA and produces temporary double-strand breaks, thus creating atransient gate (cleavage complex) through which another DNA duplex can pass. After strand passage the break is ligated and the DNA structure is restored. Numerous compounds are knownto disrupt the DNA breakage–reunion cycle of mammalian topoisomerase II. This disruption during DNA transcription or replication can result in DNA strand breaks being exposed and this may lead to clastogenicity and/or cytotoxicity if the exposed DNA strand breaks are not repaired (Lynch et al., 2003).
The comet assay is currently extensively used in in vivo and in vitro studies for the evaluation of the genotoxic potential of a variety of toxic agents such as chemical compounds, ionizing radiation and UV radiation, as well as the potential of chemical compounds such as fluoroquinolonic antibiotics (Garaj-Vrhovac and Zeljezic, 2004).
Sanchez et al. (2005) were evaluated the genotoxic potential of the three antimicrobials quinolones ofloxacin, nalidixic acid and ciprofloxacin frequently used in therapy upon irradiation with UV light by using the comet assay on cells of the Jurkat cell line (an immortalized line of T lymphocyte cells).The results demonstrate that there are significant differences between the control groups with naldixic acid and ciprofloxacin, while ofloxacin, levofloxacin is the L-isomer ofloxacin, irradiation decreases the damage slightly although the difference is not statistically significant. Presumably, the resulting photoproducts are of low toxicity and low photogenotoxicity. Although ofloxacin may generate a carbene (any member of a class of highly reactive molecules containing divalent carbonatoms) or singlet oxygen, the efficiency of generation of these may be much lower. Indeed, work in progress has shown that the presence of an alkoxy substituent (methoxy group) at position 8 reduces both the photolability and the phototoxicity Itoh et al. (2006) investigated the genotoxic potential of eight quinolones, namely nalidixic acid (NA), pipemidic acid (PPA), oxolinic acid (OA), piromidic acid (PA), enoxacin (ENX), ofloxacin (OFLX), norfloxacin (NFLX) and ciprofloxacin (CPFX), by the in vitro alkaline single-cell gel electrophoresis (comet) assay at pH>13. WTK-1 cells (mutant p53) were treated with each of the eight quinolones at 62.5-1000 µg/mL for 2, 4 and 20h. NFLX and CPFX significantly induced DNA damage concentration-dependently after 4 and 20h treatment, but this damage was recoverable. On the other hand, DNA was not damaged in the cells treated with six other quinolones.
Dwivedi et al. (2014) found, generally drugs and chemicals induce their photocytotoxicity via DNA damage, cell cycle arrest and apoptosis. Also, investigated the effect of lower concentration of photosensitized and non-photosensitized ofloxacin 25µg/ml in vitro on cell cycle progression of immortalized human keratinocyte cell line (HaCaT). Photosensitized OFLX induced cell cycle arrest in G2 phase with the progression of cells in sub-G1 phase, advocated the induction of apoptosis. DNA damage is inducer of p21 gene which prevents the activation of cyclin B1-cdc2 complexes or by blocking inhibitory phosphorylations of pocket proteins by (Cyclin B1 and cytochrome c protein) CDKs and arrest cells in G2/M phase. The up regulation of p21 by photosensitized OFLX plays a pivotal role in G2 ⁄ M cell arrest and in the induction of apoptotic pathway.
DNA damage in proliferating cells activates a pathway which arrests cell division to allow either DNA repair or induction of cell death by apoptosis.
In our study, focusing on how the comet assay has been applied to study of DNA damage and repair in rat’s stem cells that extracted from bone morrow after the same protocol of treatment that followed in the toxicological study. There was a significant P<0.05 decreases of the high DNA damage level in both treated groups, after 4 weeks and one week of withdrawal period of levofloxacin dosing in group 1 treated with therapeutic dose of levofloxacin 7.5mg/kg/b.w, while in group 2 treated with double therapeutic dose of levofloxacin 15mg/kg/b.w, was more effective in decreasing of DNA damage after 2 and 4 weeks treated with drug in comparison with control group. This result was agreed with previous results of (Sanchez et al., 2005; Itoh et al., 2006; Dwivedi et al., 2014) which that revealed the decrease DNA damage by using fluoroquinolones. So the thought is, levofloxacin is non genotoxic agent when we observed the decreases in DNA damage along experiment periods.
In this study has linked the induced apoptosis, which was markedly increased by levofloxacin in a dose-dependent manner especially in double therapeutic doses 15mg/kg/b.w. simultaneously, levofloxacin decreased cell binding to type II collagen (COL2). Thus, levofloxacin-induced apoptosis exhibits characteristics of anoikis (the process by which cell death is triggered by separation from the extracellular matrix D27), which contains COL2. At last, levofloxacin increased the ratio of Bax (an apoptosis promoter) to Bcl-2 (an apoptosis inhibitor) ratio and active caspase-3 in a dose-dependent manner. Levofloxacin therefore increases the effects of serum deprivation on anoikis by down regulating COL2 in rat via Baxto Bcl-2 /caspase-3 pathway (Yanez et al., 2012).
Also, fluoroquinolone compounds have been reported to have an inhibitory effect on cell proliferation and induce apoptosis in DNA damaged cell lines (Kumar et al., 2012; Mondal et al., 2004). In this study, comet assays indicated that levofloxacin inhibited the proliferation of rat’s stem cells extracted from bone morrow in a dose- and time-dependent manner but did not appear to disturb the proliferation of non-damaged lymphocytes as matched with the results were observed in NDI and MI values. The result provided the evidence at levofloxacin selectively suppresses damaged cell proliferation. Thus, the thought that levofloxacin could be an effective candidate for therapy against effected cells. Antibacterial fluoroquinolones are a class of antibacterial agents that are commonly used to treat human and animal infections.
The treatment of bacterial infection inhibits bacterial DNA gyrase by a mechanism similar to that of certain antitumor drugs against mammalian topoisomerase II. Some antibacterial fluoroquinolones, such as ciprofloxacin, ofloxacin and norfloxacin, also demonstrate a slight interaction with mammalian topoisomerase II, although these antibacterials are much more selective for bacterial DNA gyrase (Sun et al., 2013).
Fluoroquinolone antibacterials share a similar mechanism of action with several clinically relevant antitumor agents, such as ellipticine and etoposide. They bind to the topoisomerase II–DNA cleavage complexes, thus converting topoisomerase II into a physiological toxin that creates protein-linked DNA breaks in the genome of treated cells (Nitiss, 2009). Levofloxacin increased topoisomerase II-mediated super coiled pBR322 DNA breaks but inhibited topoisomerase II-mediated DNA religation. The effects of levofloxacin on the topoisomerase II-mediated DNA cleavage/religation were similar to that of etoposide. These findings provide evidence that levofloxacin is a poisonous inhibitor for topoisomerase II (Elsea et al., 1993). Levofloxacin binds the reversible complex between DNA and topoisomerase II, preventing the dissociation of the DNA–topoisomerase II complex and thereby inducing DNA damage. It has been reported that the key responses of fluoroquinolone-induced DNA damage is causing cell-cycle arrest and apoptosis of the treated cells (Walsby et al., 2010; Yogeeswari et al., 2005). Previous studies have shown that fluoroquinolone compounds induced G2/M cell cycle arrest and apoptosis in a variety of carcinoma cell lines as well. Cyclin B1 plays an essential role in G2/M transition of mitosis in cell proliferation (Aranha et al., 2003; Huang et al., 2011) and according to this, it supports the founded results of this study through the decreases in cell proliferation indicated by NDI and MI values and also when they regain to normal values after one week of levofloxacin withdrawal.
Aranha et al. (2003) and Huang et al. (2011) found that the treatment with levofloxacin significantly decreased the expression of cyclin B1/CDK1 and the effects of fuoroquinolone compounds on cyclin B1 down-regulation, G2/M cell cycle arrest and induction of apoptosis.
Apoptosis may be initiated by the stimulation of death receptors located on the cell surface or through an intrinsic pathway involving the release of apoptotic signals from mitochondria (Plati et al., 2011; Mason and Rathmell, 2011). The cascading activation of caspases and the release of cytochrome C from the mitochondria play key roles in apoptosis, and the type of intracellular apoptotic pathways involved may be deduced from the activated initiator caspases. It specifically investigated the mitochondria-related events during apoptosis, such as the breakdown of the mitochondrial membrane, the expression of Bax and Bcl-2, and the activation of caspase-9. Members of the Bcl-2 protein family play an important role in apoptosis by regulating the release of cytochrome C from the mitochondria to the cytosol. It has been shown that anti-apoptotic proteins, such as Bcl-2 and Bcl-XL, inhibit cytochrome C release whereas pro-apoptotic members, such as Bax, promote cytochrome C release, leading to the initiation of apoptosis (Ko et al., 2011).
Here, observed that levofloxacin mediated an up-regulation of Bax and down-regulation of Bcl-2 to induce apoptosis, possibly through increased caspase activity and by preventing the formation of anti-apoptotic bodies. Therefore, it is possible that levofloxacin induced the opening of the mitochondrial permeability transition pore through the up-regulation of Bax, resulting in the release of cytochrome C (Zhao et al., 2010; Hsu et al., 2009). In fact, Levofloxacin-induced decrease of the mitochondrial membrane potential in lymphocytes cells, followed by increased cytochrome C release from the mitochondria into the cytosol. In the mitochondrial apoptotic pathway, the release of cytochrome C is a critical event because cytochrome C forms a complex with procaspase-9 in the cytoplasm (resulting in the activation of procaspase-9), which will eventually lead to the activation of caspases-3 and the induction of apoptosis (Patel et al., 2010). Because caspase-8, when activated by the death receptor, is able to cleave the proapoptotic Bcl-2 family member and then trigger a distinct apoptotic pathway involving mitochondria in some fluoroquinolone compound-treated cell types, it is possible that the activation of caspase-9 in rat’s stem cells may be due to the activation of the death receptor-caspase-8 pathway (Chang et al., 2009; Herold et al., 2002). The thought, that the cleavage of caspase-8 was increased significantly in treated animal with double therapeutic dose 15mg/kg/b.w. Therefore, it is likely that the activation of caspase-9 is triggered mainly by the caspase-8 pathway. However, this needs further evaluation by using caspase inhibitors (Wang et al., 2008).
In conclusion, levofloxacin with different concentrations exerted potent and selectively cytotoxic activity through the mechanism of eukaryotic topoisomerase II poisoning. The growth inhibition was in large part mediated via apoptosis-associated mitochondrial dysfunction and down regulation of Bcl-2 signaling pathways. Levofloxacinmay be therefore, has a potential use as a suggestive chemotherapeutic agent in the treatment of solid cancers.
Consequently, obtained data demonstrated that levofloxacin is a cytotoxic but non genotoxic through obvious founded data within treated groups which had been decreased in CA, MI and NDI along test period which mean DNA got repaired and back to normal structure as it compared with control group, with no revealed changes seen on MNi along test period as compared with control group.
Conclusion
Levofloxacin is nongenotoxic but its cytotoxic agent and most cytogenetic effects of levofloxacin in rats with therapeutic and double therapeutic doses are reversible.
Acknowledgment
Prays is to almighty Allah, the most merciful for giving me strength to accomplish this work. My deep gratitude should get to all members of Department of the Medical and Biological Supervision / Veterinary Directorate-IRAQ for their help and encouraging me.
Conflict of Interests
There is no conflict of interest.
Authors Contribution
Regarding to the widely uses of levofloxacin in curing various microbiological diseases and its mechanism of action through inhibition DNA gyrase in prokaryotic and eukaryotic cells, it came interest to screening the cytogenetic effects in rodent model (Rat).
References