Advances in Animal and Veterinary Sciences
Research Article
Prevalence and Molecular Identification of Haemoprotozoan Diseases of Cattle in Bangladesh
Md. Akramul Bary1, Md Zulfekar Ali2, Sharmin Chowdhury1, Abdul Mannan3, Md. Nur E Azam1, Mohammad Moktader Moula4, Zafar Ahmed Bhuiyan4, Md. Taohid Wasim Shaon4 Mohammad Alamgir Hossain1*
1Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University, Chattogram -4225, Bangladesh; 2Animal Health Research Division, Bangladesh Livestock Research Institute (BLRI), Savar, Dhaka-1341, Bangladesh; 3SAQ Teaching Veterinary Hospital, Chittagong Veterinary and Animal Sciences University, Chattogram -4225, Bangladesh; 4Nourish Poultry and Hatchery Ltd. Dhaka, Bangladesh.
Abstract | A total 300 blood samples were randomly collected (150 crossbred and 150 local cattle) in three consecutive seasons (summer, rainy and winter) from four selected areas, namely Nasirabad, Patia, Bayezid and Jointika under Chittagong district of Bangladesh. The effects of topography, season, age and gender were tested in both crossbred and local cattle. The PCR was performed after consequence screening by light microscopy, which exhibited that 22 samples (14 Anaplasmaspp, 6 Babesia spp and 2 for mixed infections) were positive. The overall prevalence of haemoprotozoan diseases were 9.33% in crossbred and 5.33% in local cattle, among these babesiosis, anaplasmosis were recorded 2.66% and 6.00% in crossbred cattle and 1.33% and 3.33% in local cattle, respectively. The highest prevalence of anaplasmosis was found in Patia (9.33%) followed by Bayezid (4.00%), Nasirabad (2.67%) and Jointika (2.66%) and babesiosis was recorded in Bayezid (4.00%) followed by Jointika (2.66%) and Patia (1.33%). Among three seasons the highest prevalence of anaplasmosis was recorded 12.00% in crossbred cattle followed by 6.00% in local cattle in summer whereas babesiosis was highest in summer (4.00%) in crossbred cattle followed by 2.00% in local cattle. Prevalence of anaplasmosis increased significantly (P<0.05) with the increase of age in crossbred cattle. the highest prevalence of anaplasmosis was 13.72% and 6.94% in adult crossbred and local cattle, respectively. Occurrence of babesiosis was the highest in adult (5.88%) in crossbred than young (2.78%) in local cattle, respectively. It was revealed that haemoprotozoan diseases were more common in female cattle, among these highest prevalence of anaplasmosis was recorded (6.11%) in female crossbred cattle and (4.00%) in local cattle, respectively. Positive samples were analyzed by PCR, where 9 samples were amplified among these 4 samples (1.33%) of Babesia spp and 5 samples (1.67%) of Anaplasma spp.
Keywords | Crossbred, local cattle, Prevalence, Haemoprotozoan, PCR
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | March 01, 2018; Accepted | April 18, 2018; Published | April 21, 2018
*Correspondence | Mohammad Alamgir Hossain, Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University, Chattogram-4225, Bangladesh; Email: hossainalamgir54@yahoo.com
Citation | Bary MA, Ali MZ, Chowdhury S, Mannan A, Nur e Azam M, Moula MM, Bhuiyan ZA, Shaon MTW, Hossain MA (2018).Prevalence and molecular identification of haemoprotozoan diseases of cattle in Bangladesh. Adv. Anim. Vet. Sci. 6(4): 176-182.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.4.176.182
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Bary et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Livestock is one of the most prospective sub sectors of agriculture in Bangladesh which plays an indispensible role in upholding human health and national economy of the country. Livestock not only assists to upgrade the financial condition but also makes substantial contribution to human nutrition. However livestock is an integral part of farming system which has a better contribution to enhance the economy of Bangladesh. The total contribution of livestock sub-sectors to gross domestic products (GDP) in Bangladesh is approximately 1.78% and in agricultural products 13.3% (BBS, 2017). It also generates 13% of foreign currency and provides 20% fulltime employment and 50% partial employment of rural population (Khokon et al., 2017).Vector-borne diseases especially babesiosis, anaplasmosis and theileriosis is distributed worldwide. These diseases are considered as one of the major obstacle and burning veterinary problems in health and productive performance of cattle (Rajput et al., 2005; Kuttler, 2018). These diseases causes devastating looses to livestock industry throughout the world (Ananda et al., 2009) as they have got a serious economic impact due to obvious reason of death, decreased productivity, declined working efficiency and limits the introduction of genetically improved cattle in an area (Uilenberg, 1995). In Bangladesh 80% rural people rear indigenous cattle (Siddiki et al., 2009) and many people are dependable on dairy farming under the traditional husbandry practices. But the production and productivity of animals are greatly hampered by different diseases including haemoprotozoan diseases (Ngole et al., 2004). Cattle infected with haemoprotozoan diseases are difficult to detect because of the low numbers of parasites that occur in peripheral blood. However, diagnosis of low-level infections with the parasite is important for epidemiological studies (Fahrimal et al., 1992). PCR has proven to be very sensitive particular in detecting Babesia bovis and Babesia bigemina in cattle (Calder et al., 1996).To overcome the economic losses early proper diagnosis of haemoprotozoan diseases are important in cattle. The present study was aimed at the molecular detection of major important blood protozoa are Babesia spp, Theileria spp and Anaplasma spp by PCR technique for the early and accurate diagnosis of haemoprotozoan diseases in cattle and to differentiate Babesia spp and Anaplasma spp by using species primers and to study the epidemiological pattern of this disease in different agro-climate zones of Chittagong district under consideration of age, sex, breed, season for determination of prevalence of haemoprotozoan diseases in cattle.
MATERIALS AND METHODS
Study Design
The study was conducted in topographically four different areas of Chittagong district namely Jointika, Nasirabad, Bayezid and Patia. The study was undertaken for a period of 12 months starting from January’ 2014 to December’2014; where three seasons were included as summer (March to May), Rainy season (June to august) and winter season (October to December). Crossbred and local cattle(Indigenous/ non descriptive/ Red Chittagong cattle) were selected for this study as target animals.
Experimental Layout
To determine the age and susceptibility for blood protozoa, cattle are categorized in the sub groups. The age limit for Holstein Friesian (HF) crossbred cattle was calf (≤1 year), young (>1–< 2.5 year) and adult (≥2.5years). In local cattle, the age limit differs for calf (≤1 year), young (>1-3.5 years) and adult (≥3.5 years) only. In each season, 50 HF crossbred cattle were considered where 51 adult, 36 young and 63 calves were taken from different areas of farms of Chittagong district. On the other hand, 50 indigenous cattle were 72 adult, 36 young and 42 calves were taken from four different areas of Chittagong district. Samples were collected randomly in three consecutive seasons; summer (March to May), rainy (June to August) and winter (October to December). A total 300 blood samples were collected for this study.
Sample Collection and Microscopic Examination
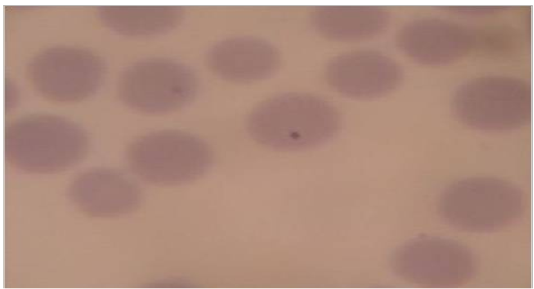
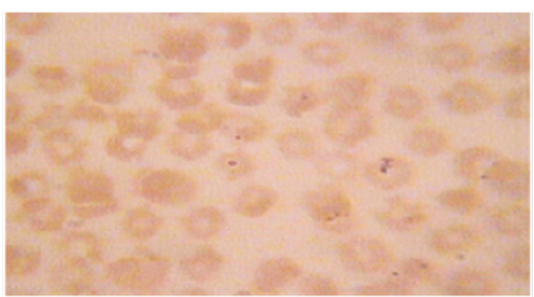
A total 300 clinical cases of Cross bred and local cattle with clinical symptoms such as anorexia, pyrexia, pale mucous membrane were screened for haemoprotozoan parasites diseases. Approximately 3-5 ml blood sample was collected during this study from an individual animal from jugular vein using 10 ml disposable plastic syringe from each animal and then preserved in BD Vacutainer® tube containing anticoagulant (Lithium Heparin).Then prepared thin blood smears were stained with the Giemsa stain for 25-30 minutes. After rinsing with water, the stained blood smears were air dried and examined under a binocular microscope (×100) with immersion oil for the identification of blood protozoa, shown in Figure 1 and 2 (Hendrix and Robinsin, 2006).
Table 1: Primers were used for PCR amplification as per the details given in the following
| Sl. No. | Gene | Primer name | Sequences (5ʺ-3") | Base pair (bp) | Reference |
| 1 | 18S rRNA | Bab- F | GTTTCTGMCCCATCAGCTTGAC | 422-440 |
Hilpertshauser et al. (2006) |
| Bab- R | CAAGACAAAAGTCTGCTTGAAAC | ||||
| 2 | 16S rRNA | AE1-F | AAGCTTAACACATGCAAGTCGAA | 1406-1422 |
Kim et al. (2010) |
| AE1-R | AGTCACTGACCCAACCTTAAATG |
DNA Extraction from Blood Sample
Total genomic DNA has been extracted from the whole blood samples by using PCI (Phenol, Chloroform and Isoamyl alcohol) method (Sambrooket al.,1989).
PCR Detection Assay for Anaplasma spp
To detect the 16S rRNA of Anaplasma spp, PCR was conducted from positive samples by using primer sets and PCR reaction mixture was performed using the commercially available Intron® PCR 2× master mix and primer (Table 1 and 2). To identify the Anaplasma from DNA samples,16S rRNA gene amplifications were performed at the following thermal conditions: 94°C for 5 min, followed by 40 cycles of 94°C for 1 min, 59°C for 1 min, 72°C for 2 min and followed the final extension step at 72°C for 10 min.
PCR Detection Assay for Babesia spp
To detect the 18S rRNA of Babesia spp., PCR reaction mixture was performed using the commercially available Intron® PCR 2x master mix and primer (Table 1 and 2). Fragment size is 422-440bp and.18S rRNA gene amplifications were performed at the following thermal conditions 94°C for 3 min, followed by 40 cycles of 94°C for 30 sec, 61°C for 30 sec, 72°C for 45 sec and followed the final extension step at 72°C for 10 min.
Table 2: Composition of reaction mixture for PCR
| Sl. No. | Components | Quantity | Final concentration (20 µl) |
| 1 | Promega PCR master mix | 10 µl | |
| 2 | Forward Primer (10 pmole/μl) | 1μl | 10 pmole |
| 3 | Reverse Primer (10pmole/μl) | 1 μl | 10 pmole |
| 4 | Water | 6 µl | |
| 5 | DNA Template | 2 µl | |
| Grand Total volume | 20 µl | ||
Gel Documentation
The 1.5% agarose gel was made by using 0.5 gm agarose powder and 50ml TAE buffer with ethidium bromide. The DNA amplicons were visualized using 4 μl of the final PCR product and 2 μl standard 100bp with DNA markers (Invitrogen®) at 120 V/100mA for 30 min. Positive or negative amplifications were evaluated as presence or absence of visible orange color bands on agarose gels under UV light (O’Dwyeret al., 2008).
Statistical Analysis
The obtained data were analyzed by using Microsoft Excel-2007 to STATA/IC-11.0 software. Descriptive statistics were expressed as proportion with confidence interval. The result was expressed in percentage with p value for Chi-square test was used to evaluate the hypothesis for significant difference between the infection in different locations and cattle breed. P values less than 0.05 (P<0.05) was considered statistical significant.
Table 3: Overall prevalence of haemoprotozoan diseases on the basis of Microscopic examination
|
haemoprotozoan Diseases |
Crossbred cattle (n=150) |
Local cattle (n=150) |
||
| (%) | 95% CI | (%) | 95% CI | |
| Babesiosis | 2.66 | 0.07-6.68 | 1.33 |
0.16-4.73 |
| Anaplasmosis | 6.00 | 2.78-11.08 | 3.33 | 1.07-7.6 |
| Mixed infection | 0.67 | 0.016-3.65 | 0.67 | 0.016-3.65 |
| Overall | 9.33 | 5.19-15.16 | 5.33 |
2.33-10.23 |
RESULTS
Prevalence of Haemoprotozoan Diseases
The overall prevalence of haemoprotozoan diseases was 9.33% (CI: 5.19-15.16) in crossbred and 5.33% (CI:2.33-10.23) in local indigenous cattle. The overall prevalence was recorded in anaplasmosis which was 6.00% (CI: 2.78-11.08) and 3.33% (CI: 1.07-7.6) for crossbred and local cattle, respectively (Table 3). Occurrence of babesiosis was 2.66% (CI:0.07-6.68) in crossbred and 1.33% (CI: 0.16-4.73) in local cattle. The highest prevalence of anaplasmosis was recorded 12.00% in crossbred cattle followed by 6.00% in local cattle in summer (Table 4). Frequency of babesiosis was highest in summer which was 4.00% in crossbred cattle followed by 2.00% in local cattle. Prevalence of Anaplasmosis and Babesiosis in local cattle was negative in winter season. it was also observed that prevalence of anaplasmosis increased significantly (P<0.05) with the increase of age in crossbred cattle (Table 5). the highest prevalence of anaplasmosis was 13.72% and 6.94% in adult crossbred and local cattle, respectively. it was also recorded that prevalence of babesiosis increased with the increase of
Table 4: Seasonal prevalence (%) of haemoprotozoan diseases
|
haemoprotozoan diseases |
Crossbred cattle | Local cattle | |||||||
| Seasons | Seasons | ||||||||
|
Summer (n=50) |
Rainy (n=50) |
Winter (n=50) |
P Value |
Summer (n=50) |
Rainy (n=50) |
Winter (n=50) |
P value |
||
| Babesiosis | 4.00 | 2.00 | 2.00 | 0.36 | 2.00 | 2.00 | 0.00 | 0.60 | |
| Anaplasmosis | 12.00 | 4.00 | 2.00 | 0.14 | 6.00 | 4.00 | 0.00 | 0.60 | |
| Mixed infection | 0.00 | 2.00 | 0.00 | 0.36 | 2.00 | 0.00 | 0.00 |
0.37 |
|
Table 5: Age specific prevalence (%) of haemoprotozoan diseases
|
haemoprotozon diseases |
Crossbred cattle | Local cattle | ||||||
| Age Group | Age Group | |||||||
|
Calf (n=63) |
Young (n=36) |
Adult (n=51) |
P value |
Calf (n=42) |
Young (n=36) |
Adult (n=72) |
P value |
|
|
Babesiosis |
1.58 | 0.00 | 5.88 | 0.42 | 0.00 | 2.78 | 1.38 | 0.73 |
| Anaplasmosis | 1.59 | 2.78 | 13.72 | 0.016 | 0.00 | 0.00 | 6.94 | 0.12 |
| Mixed infection | 1.58 | 0.00 | 0.00 | 0.50 | 0.00 | 0.00 | 1.38 |
0.78 |
Table 6: Sex-specific prevalence (%) of haemoprotozoan diseases
|
haemoprotozoan diseases |
Crossbred cattle | Local cattle | ||||
|
Male (n=21) |
Female (n=131) |
P value |
Male (n=25) |
Female (n=125) |
P Value |
|
| Babesiosis | 0.00 | 3.05 | 0.47 | 0.00 | 1.60 | 0.67 |
| Anaplasmosis | 4.76 | 6.11 | 0.43 | 0.00 | 4.00 | 0.39 |
| Mixed infection | 0.00 | 0.76 | 0.92 | 0.00 | 0.80 |
0.91 |
Table 7: Location specific prevalence (%) of haemoprotozoan diseases
|
haemoprotozon diseases |
Nasirabad | Patia | Bayezid | Jointika |
| Babesiosis | 0 | 1.33 | 4 | 2.66 |
| Anaplasmosis | 2.67 | 9.33 | 4 | 2.67 |
| Mixed infection | 1.33 | 0 | 1.33 |
0 |
Table 8: Overall prevalence of haemoprotozoan diseases on the basis of PCR
|
haemoprotozoan Diseases |
Crossbred cattle (n=150) | Local cattle (n=150) | ||
| (%) | 95% CI | (%) | 95% CI | |
| Babesiosis | 1.33 | 0.16-4.73 | 1.33 | 0.16-4.73 |
| Anaplasmosis | 2.00 | 0.41-5.7 | 1.33 | 0.16-4.73 |
| Mixed infection | 0.00 | 0.00 | 0.00 | 0.00 |
| Overall | 3.33 | 1.00-7.6 | 2.66 |
0.07-6.68 |
age. In this investigation, it was revealed that Female are more prone to haemoprotozoan diseases than male cattle. Higher prevalence of anaplasmosis was recorded in female crossbred cattle which was 6.11%, male crossbred cattle showed 4.76% and have not recorded babesiosis in local male cattle and prevalence of babesiosis was recorded in female local cattle which 1.60% (Table 6). In crossbred and local cattle, Anaplasmosis was consistently prevalent in all the study areas and babesiosis also prevalent all study areas except Nasirabad (Table 7).
Molecular Identification of Haemoprotozoan Diseases
Microscopy detected a total 22 positives (7.33%) samples, 14 samples for Anaplasma spp, 6 samples for Babesia and 2 samples for mixed infections were identified, respectively which was confirmed in 9 samples (3.00%) by PCR technique by using 18s rRNA gene of Babesia and 16s rRNA gene of Anaplasma species (Table 8; Figure 3 and 4). Microscopy detected 13 (4.33%) showed negative result in PCR.
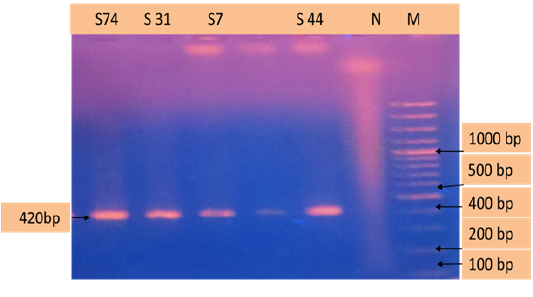
Figure 3: PCR products amplified using bab-F & bab-R specific primers. Lane M: 100 bp DNA ladder, lane N: negative control, lane: S74, S31, S7,S44 positive samples.
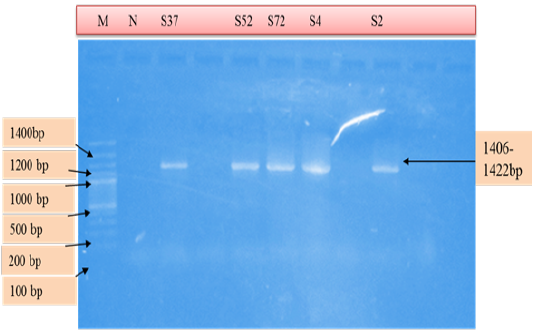
Figure 4: PCR products amplified using AE1-F & AE1-R specific primers. Lane M: 100 bp DNA ladder, lane N: Negative control, lane: S37, S52, S72, S4, S2 positive samples.
DISCUSSION
The overall prevalence of haemoprotozoan diseases was 3.00% by PCR which Babesia spp (1.33%) was poorer than Terkawi et al. (2011) where overall prevalence of Babesiabovis and Babesia bigemina was 11.2% and 3.6% by PCR in Thailand. The overall prevalence of haemoprotozoan diseases in the present study on the basis of microscopic examination (7.33%), which were inferior than those of Kamani et al. (2010) and Khan et al. (2004), who recorded 25.7% in North-Central Nigeria and 22.31% and 27.69% in Pakistan, respectively. Ananda et al. (2009) and Chowdhury et al. (2006) also documented higher prevalence of haemoprotozoan diseases in clinically susceptible cattle in India and Bangladesh, respectively. Lower prevalence of haemoprotozoan diseases in the present study might be due to random sampling, variation in geo-climatic condition, breed and exposure of vectors and age of the animals might contribute to variable prevalence of haemoprotozoan diseases in the study areas (Khan et al. 2004). It was observed that haemoprotozoan diseases occurred more in crossbred than local cattle which was partially consistent by the earlier reports of Soulsby (1982), who reported that exotic breeds (Bos taurus) showed more susceptibility to haemoprotozoan diseases than local breeds (Bos indicus).constant exposure of infections and development of immunity against such infections might responsible for lower prevalence in local cattle (Siddiki et al., 2009). On the contrary, more attention in the management of HF crossbred cattle give less chance of pre exposure of vectors and develop no or less immunity resulting frequent occurrence of such diseases (Chowdhury et al., 2006).
The prevalence of Babesiosis of this study was related to the observation revealed by Siddiki et al. (2009); Chowdhury et al. (2006),who recorded 1%, 3.3% and 2.29% infections, respectively in different areas of Bangladesh. However, the prevalence of Babesiosis in local cattle was lower than those of Kalkan et al. (2010); Khan et al. (2004) and Savini et al. (1999), who recorded 5.83% in Turkey, 5.5% in Pakistan and 4.95% in Italy, respectively. The overall prevalence of Babesiosis in this investigation was poorer with the earlier report of Alim et al. (2012) who recorded 7.14% in indigenous cattle and 9.25 % in cross breed cattle in Chittagong. The overall lower prevalence of Babesiosis of the current study which might be due to variation of study areas or unavailability of tick vectors.
Prevalence of anaplasmosis of this study was advanced with the reports of Siddiki et al. (2009) and consistence with the reports Samad et al. (1989), who recorded 3% and 5.93% in different areas of Bangladesh. Higher prevalence of Anaplasmosis was clarified as endemicity of such diseases in those areas. The occurrence of haemoprotozoan diseases vary greatly according to seasons. observation of summer season of this research was in accordance with the report of Ananda et al. (2009). In the present study, it was observed that haemoprotozoan diseases occurred more in summer season. The highest prevalence of Anaplasmosis was recorded 12.00% in crossbred cattle followed by 6.00% in local cattle in summer. Frequency of babesiosis was highest in summer which was 4.00% in crossbred cattle followed by 2.00% in local cattle. lower temperature and humidity of winter months were less favorable for the growth and multiplication of tick vectors which might contribute to lower frequency of such diseases in the study population (Zahid et al., 2005).
Age also inspirations the occurrence of haemoprotozoan diseases. In the current study, higher susceptibility of adult cattle to haemoprotozoan diseases were found consistent with the findings of of Khan et al. (2004), who reported highest prevalence in animals aged more than 3 years followed by the lowest prevalence in less than 1 year of age. Ananda et al. (2009) also documented higher prevalence in animals aged more than 3 years followed by the lower prevalence in 1-2 years of age. observation of this study also supported by the findings of Khan et al. (2004) and Kamani et al. (2010), who observed higher prevalence in adult cattle (36.3%) than young-stock (30.76%).
In the current study, higher prevalence of Anaplasmosis in adult followed by young cattle was in accordance with the reports of Chakraborti (2002). The earlier researchers reported that calves up to 9 months or even 1 year of age usually showed no clinical illness whereas cattle 1 to 2 years or above 3 years of age may develop acute or fatal form of disease. Observation of this study also showed consistence with the findings of Chowdhury et al. (2006) and Chakraborti (2002), who observed comparatively higher prevalence in adult than calves.
Femininity of animals also has influences in the occurrence of haemoprotozoan diseases. The prevalence of such disease in female animals of this study showed consistency with the observation of Kamani et al. (2010), who recorded comparatively higher prevalence in female than male cattle in Nigeria. In this study, higher prevalence in female cattle might be due to stress condition as they were kept longer period for breeding and milk production purpose or supplied imbalance diet against their high demand (Kamani et al., 2010).
Microscopy detected a total of 22 positives (7.33%) samples which was confirmed in 9 samples (3.00%) by PCR technique. Fahrimal et al. (1992) who studied the carrier cattle infected with Babesia spp by using PCR amplification of a portion of gene from the parasite. Hermans et al. (1994) in Costa Ricaw here they assessed the infection of Babesia which transmitted from the ticks by the use of PCR analysis. Serological methods are employed in diagnosing subclinical infections in epidemiological studies, but false-positive and false negative results may be done due to cross-reactions or weakening of specific immune responses. They serve as reservoirs of infection for ticks and cause natural transmission of the disease (Calder et al., 1996).
CONCLUSION
haemoprotozoan diseases were strongly associated with age of animals, cattle of any age could be affected by haemoprotozoan diseases but inverse age resistance was noticed in the occurrence of babesiosis where crossbred cattle were more susceptible than local. Babesiosis and Anaplasmosis were predominant diseases in the study areas. Frequency of blood protozoan infections increased with weather change where summer season was found most vulnerable. Finally, molecular identification of haemoprotozoan diseases by PCR technique for the early and accurate diagnosis of haemoprotozoan diseases in cattle and to differentiate Babesia spp and Anaplasma spp by using species primers for taking further control strategies in the study areas.
ACKNOWLEDGEMENTS
Authors would like to greatly acknowledge the financial support provided by UGC-HEQEP round-II sub project (CP:2180), Bangladesh. We are also thankful to all of my faculty’s teachers and friends of CVASU for their support and inspiration during the project tenure.
Conflict of interest
We don’t have any conflict of interest.
Authors Contribution
MAB, MZA, SC, AM and MAH has made substantial contribution to conception and design, acquisition of data, analysis and interpretation of data. MNA, MMM, ZAB and MTWS carried out drafting the manuscript. All authors read and approved the final manuscript.
References