Advances in Animal and Veterinary Sciences
Short Communication
Variation of Tannin Contents in Selected Agro-Industrial By-products and their Biological Activity in Precipitating Protein
Makoto Kondo1*, Anuraga Jayanegara2, Yutaka Uyeno3, Hiroki Matsui1
1Graduate school of Bioresources, Mie University, Kurimamachiya, Tsu city, Mie, Japan, 514-8507; 2Faculty of Animal Science, Bogor Agricultural University, Bogor 16680, Indonesia; 3Faculty of Agriculture, Shinshu University, Minamiminowa, Nagano, Japan.
Abstract | Agro-industrial by-products containing tannins have potential to be used as alternative ruminant feed sources. To explore the functional effects of agro-industrial by-products on livestock production, the contents of tannins such as total extractable phenolics (TEPH) and condensed tannins (CT), in 15 kinds of by-products were determined and their protein-precipitating capacity (PPC) was also measured. TEPH content was the highest in chestnut husk, followed by tea grounds. Grape skin, winery residue and chestnut husk showed high CT content. Furthermore, TEPH, but not CT, content was positively correlated with PPC (p<0.001). The PPC on a TEPH basis was the highest in black tea grounds, followed by cedar bark and cypress bark. Green tea grounds, banana peel, grape skin, and winery residue showed moderate PPC, whereas citrus peels and coffee grounds did not show any PPC. Among these by-products, four by-products showing moderate PPC might have potentiality to be considered as feedstuffs that protect protein degradation in the rumen; particularly green tea grounds would be preferred as protein supplements due to high CP content and moderate PPC.
Keywords | Agro-industrial by-products, Phenolics, Protein precipitating capacity, Tannins
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 24, 2015; Revised | November 12, 2015; Accepted | November 15, 2015; Published | December 23, 2015
*Correspondence | Makoto Kondo, Mie University, Kurimamachiya, Tsu city, Mie, Japan; Email: [email protected]
Citation | Kondo M, Jayanegara A, Uyeno Y, Matsui H (2016). Variation of tannin contents in selected agro-industrial by-products and their biological activity in precipitating protein. Adv. Anim. Vet. Sci. 4(2): 66-70.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.2.66.70
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Kondo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Global consumption of animal products such as dairy and meat has been increasing; as a result, demands for livestock feedstuffs has also increased. In recent years, raw material for livestock feedstuffs has witnessed competition with not only human food supply but also energy resources (Nonhebel and Kastner, 2011). Agro-industrial by-products have been studied in many countries as alternative feed sources. The non-edible parts of agricultural products are considered as by-products, and some contain considerable amounts of plant secondary metabolites such as tannins. Tannins are polyphenolic compounds present in plants and are known as anti-nutritional factors. Animals fed tannin-rich diets show decreased feed intake, increased faecal nitrogen excretion, reduced digestibility, and less ruminal degradation (Min et al., 2003). However, beneficial effects of tannins on ruminant nutrition have also been reported (Min et al., 2003). In some cases, ingestion of tannins increases amino acid absorption from small intestine, improves animal performance, reduces internal parasites, mitigates methane production from the rumen, and decreases bloating (Mueller-Harvey, 2006). To identify the characteristics of tannins, total tannin content, as well as their activity in terms of protein precipitating capacity (PPC), are important (Hoste et al., 2006; Jayanegara et al., 2015). To explore the functional effects of agro-industrial by-products on livestock production, characterization of these by-products in terms of tannins is needed. The aim of this study was to investigate the properties of 15 agricultural by-products based on total phenolic compounds, tannins, and their activity measured as PPC.
Fifteen kinds of by-products were collected from agro-industries in Japan. These included green tea, oolong tea, and black tea grounds, winery residue, coffee grounds, cacao husk, chestnut husk obtained from beverage or snack factories; orange peel, grape fruit peel, lemon peel, banana peel, grape skin were collected from fresh juice shops. Cedar bark and cypress bark were obtained from a lumber mill. Samples for analyses were prepared with freeze-drying and milling with using a 1.0 mm mesh screen. Crude protein (CP) content was determined by Kjeldahl method; acid detergent fiber (ADF) and acid detergent lignin (ADL) content were analysed as outlined by Van Soest et al. (1991). For tannin analyses, 200 mg of ground samples were extracted by 10mL of 70% aqueous acetone. Total extractable phenolics (TEPH) and condensed tannins (CT), and their PPC values in the extract were determined by the method described by Makkar (2003). PPC was measured at pH 5.0 on bovine serum albumin (BSA) containing agar plate, and the value was expressed as the amount of BSA bound to DM or TEPH content of the by-product. Analyses of CP, ADF, ADL, TEPH and CT were performed in duplicate whereas PPC determination was conducted in triplicate; the values were presented descriptively. Statistical analyses for Pearson correlation coefficients between PPC and TEPH content or CT content were tested by CORR procedure in SAS 9.3 (SAS Institute, Carry, USA).
Most of the by-products examined contained low CP (< 10 % on a dry matter (DM) basis), however, each of the tea ground had a CP value higher than 20% DM, and the green tea grounds showed the highest content. Previous studies have also shown that proteins in green tea grounds can be digested in ruminants; however, those in black tea are less digestible (Kondo et al., 2014). The varying protein digestibility among different types of tea grounds could be attributed to the different tannin characteristics of these tea grounds. ADF is a less-digestible fiber fraction, and its contents in the by-products considerably varied; for example, barks showed the highest ADF levels (almost 90% DM), followed by peanut husk. These by-products also showed high ADL levels, indicating low digestibility of constituent fibers, even for ruminants. Extractable phenolic compounds were found to be high in chestnut husk, all kinds of tea grounds, and grape skin (>7% DM). Makkar et al. (1990) reported TEPH content in some agro-industrial by-products collected in India, such as pods, kernels, and seeds from tropical agricultural products. The TEPH content was almost comparable with the by-products in the present study. Polyphenols in tea leaves has been identified— epigallocatechin, epigallocatechin gallate are major compounds in green tea, whereas theaflavins are found in black tea (Yamamoto et al., 1997). These different structures in phenolic compounds are related to their biological activity. Polyphenols in citrus has also been studied in food science. The major phenolics found in citrus are flavonoids such as hesperidin, naringin, and eriocitrin. Although phenolics are present at higher level in the peels than in flesh (Miyake et al., 1998), the phenolics levels in the peels were relatively low in the by-products tested in the present study. A lower amount of extractable phenolics was also detected in cedar bark and cypress bark. The barks contained a high amount of insoluble lignin and large-molecule polyphenolic compounds. Phenolics were expected to be present in lignin molecules, but these could not be extracted using 70% acetone in this experiment. CT content also varied among the by-products: grape skin, winery residue and chestnut husk contained more than 10% DM, whereas banana peel, peanut husk, green tea ground and oolong tea ground had moderate amount (2–4% DM). Citrus peel, coffee grounds, cacao husk, barks and black tea had less than 1% DM. Ideally, CT is a subfraction of TEPH; however, the CT level in the top three by-products was higher than total TEPH. This could be due to the uncertainty of quantifying CT. We have used the equation derived from leucocyanidin to quantify CT in these by-products, as described by Makkar (2003). This method is simple and can be applied to many plant samples; how ever, there is uncertainty with the quantification due owing to the lack of a suitable standard molecule. Purification of tannins as a standard substrate from each sample would be better means to determine tannin content (Naumann et al., 2014), but individual purification is complicated and not always a suitable approach. The quantification of CT in these by-products requires further detail analyses; however, we could identify the by-products that contain higher or lower amounts of CT with a simple assay. In the present study, we measured tannin contents in freeze-dried materials, however, tannin contents and ruminal degradability of agro-industrial by-products change during their processing such as drying and ensiling (Bagheripour et al., 2008). For a practical use of the by-products examined here, the effect of processing on tannins should be clarified.
The PPC is considered to be a measure of biological activity of tannins in animal feed (Makkar et al., 1990). Black tea grounds had the highest PPC, followed by chestnut husk, oolong tea grounds, and grape skin. Banana peel, peanut husk, cacao husk, cedar bark, cypress bark, and green tea grounds showed moderate or low PPC, whereas citrus peels and coffee grounds had negligible PPC (Figure 1, left). The tea grounds showed considerably different PPC values, which may attributed to the difference in molecular structure of tannins in tea grounds; the order of binding proteins increases as the number of OH groups in tea polyphenols increases (Ozdal et al., 2013). The PPC on a DM basis indicates how many proteins can be bound by the by-products and PPC on a TEPH basis indicates the activity of phenolic compounds themselves. The amounts and/or structures of phenolic compounds vary between the plant species, parts, growing stage, and other factors (Makkar et al., 1990; Naumann et al., 2014). The PPC expressed as a unit of TEPH gives more detail information of the activity of phenolic compounds. In this case, phenolics in black tea ground showed the highest PPC, followed by those in two kinds of barks (Figure 1, right). The high PPC in black tea grounds had also been revealed in the digestive tract of ruminant. Our previous study showed
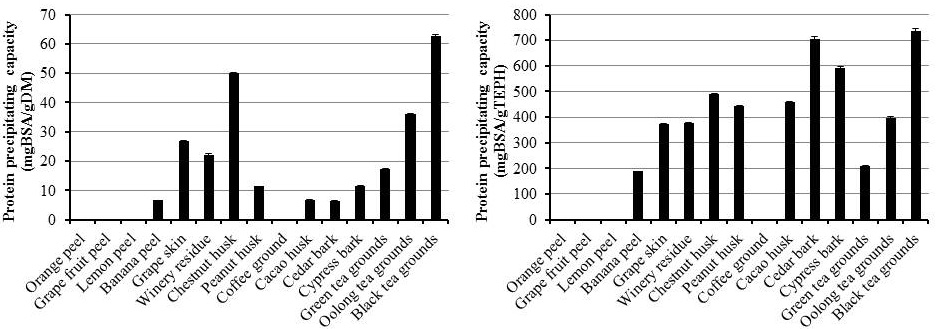
Figure 1: Protein precipitating capacity on a dry matter basis (left) and on a total extractable phenolics basis (right) in agro-industrial by-products
Each bar indicates mean ±SEM (n = 3).
that feeding of black tea grounds at a 5% DM of the total diet to goats showed lower CP digestibility and higher excretion of acid-detergent insoluble proteins; these could be interpreted as tannin-protein complexes (Kondo et al., 2007). In contrast with PPC on a DM basis, two types of barks also showed higher levels of PPC on a TEPH basis among the by-products in the present study. These results indicated that the barks contained low amounts of extractable phenolics, but these compounds could bind more protein than phenolics found in other by-products. According to comparable PPC on a TEPH basis between barks and black tea grounds, pheolics in barks possibly make complexes with proteins and the complexes would be too tight to break in digestive tract in ruminant, similar to those found in black tea grounds. The phenolics in black tea grounds and two kinds of bark could be useful for anthelmintic effect, which requires a strong protein binding capacity in the intestine (Hoste et al., 2006). Similar to the barks, phenolics in the husks from chestnut, peanut, and cacao also showed relatively higher PPC on a TEPH basis as compared with other by-products. Particularly, peanut husk and cacao husk showed higher levels of PPC when it was expressed on a TEPH basis. This result also indicates that the phenolics in these husks can bind so much protein that even the TEPH contents were low. Furthermore, banana peel, grape skin, winery residue, and green tea grounds showed low or moderate PPC on a TEPH basis. Our previous data showed that phenolics in green tea grounds protect proteins from degradation in the rumen, but do not strongly inhibit protein digestion in the post-rumen (Kondo et al., 2014). According to the present data, phenolics in banana peel, grape skin and winery residue may also bind proteins moderately in the rumen, but they may exhibit lower binding activity in post-rumen, as similar to green tea ground. Min et al. (2003) reported that moderate levels of tannins protect protein degradation in the rumen and increase amino acid absorption in the small intestine, thereby improving the growth rate in lambs. Further research is needed to determine whether the by-products with moderate PPC increase the efficiency of protein digestion in ruminants.
Figure 2 shows simple correlations between PPC on a DM basis and TEPH content (left) or with CT content (right). The correlation coefficients of PPC with TEPH and CT were 0.851 (p<0.001) and 0.468 (p=0.078), respectively. These results are similar to those observed for some agro-industrial by-products in India (Makkar et al., 1990). Significant correlation between PPC and TEPH suggests that the PPC is influenced by TEPH and not by CT. Not only content but also structures of tannins vary widely among different plant materials (Naumann et al., 2014). In addition to CT in plants, hydrolysable tannins and simple phenolic compounds such as gallates and flavonoid monomers that can be detected by TEPH assay, may bind to proteins. Parameters such as TEPH would be acceptable in understanding the general characteristics of tannins in agro-industrial by-products.
In conclusion, based on the information of the chemical compositions and PPC (Table 1), the by-products examined in the present study can be classified into 5 groups; group 1 showed high PPC both on DM and TEPH basis (black tea grounds, oolong tea grounds and chestnut husk), group 2 showed low TEPH content, but high PPC (peanut husk, cacao husk, ceder bark and cypress bark), group 3 showed high CP and moderate PPC (green tea grounds), group 4 showed low CP and moderate PPC (banana peel, grape skin and winery residue) and group 5 showed no PPC (orange peel, grape fruit peel, lemon peel and coffee grounds). For livestock production, group 1 by-products may have the potential for use as anthelmintic agents without any
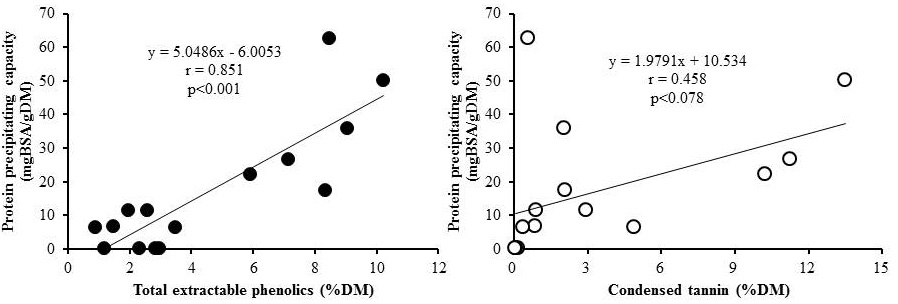
Figure 2: Correlation between protein precipitating capacity with the contents of total extractable phenolics (left) and condensed tannins (right)
BSA: bovine serum albumin
Table 1: Chemical compositions of agro-industrial by-products
|
CP (%DM) |
ADF (%DM) |
ADL (%DM) |
TEPH (%DM) |
CT (%DM) |
|
|
Orange peel |
5.7 |
16.6 |
1.6 |
2.3 |
0.1 |
|
Grape fruit peel |
7.2 |
17.6 |
1.6 |
3.0 |
0.2 |
|
Lemon peel |
9.3 |
21.5 |
5.3 |
2.8 |
0.1 |
|
Banana peel |
8.4 |
16.7 |
2.6 |
3.5 |
4.9 |
|
Grape skin |
8.7 |
45.0 |
25.0 |
7.1 |
11.3 |
|
Winery residue |
7.6 |
27.9 |
17.9 |
5.9 |
10.3 |
|
Chestnut husk |
4.0 |
51.6 |
25.4 |
10.2 |
13.5 |
|
Peanut husk |
4.4 |
77.9 |
35.9 |
2.6 |
2.9 |
|
Coffee ground |
13.6 |
42.2 |
13.7 |
1.2 |
0.0 |
|
Cacao husk |
15.6 |
38.8 |
17.6 |
1.5 |
0.9 |
|
Cedar bark |
2.7 |
88.4 |
45.8 |
0.9 |
0.4 |
|
Cypress bark |
2.5 |
90.1 |
55.3 |
2.0 |
0.9 |
|
Green tea ground |
29.2 |
24.3 |
8.7 |
8.3 |
2.1 |
|
Oolong tea ground |
22.6 |
33.6 |
16.3 |
9.0 |
2.1 |
|
Black tea ground |
25.5 |
27.1 |
9.5 |
8.5 |
0.6 |
CP: crude protein; ADF: acid detergent fiber; ADL: acid detergent lignin; TEPH: total extractable phenolics; CT: condensed tannin
pretreatment, whereas group 2 by-products may have the same potential after extraction and condensation of effective fractions. Among these by-products, four by-products in group 3 and 4 showing moderate PPC can be considered as feedstuffs that protect protein degradation in the rumen; particularly green tea grounds would be potentially preferred as protein supplements due to high CP content and moderate PPC. For the future implications of these by-products, inhibitory interactions between tannins and rumen microorganisms should also be investigated since tannins may inhibit the growth of microoganisms due to their PPC (McSweeney et al., 2001). Further researches using animal experiments are necessary to understand both positive and negative the biological activities of tannins not only on protein utilization but also on microbiome in ruminant with related to tannin contents and PPC.
ACKNOWLEDGEMENTS
This work was supported by JSPS KAKENHI Grant Number 15K07272.
Authors contribution
Makoto Kondo carried out the experiment, and wrote the draft of manuscript. Anuraga Jayanegara performed the statistics and provided technical support. Yutaka Uyeno and Hiroki Matsui checked the experimental design and supported in writing the manuscript. All authors revised the manuscript.
Conflict of Interest
The authors have no conflict of interests.
REFERENCES






