Advances in Animal and Veterinary Sciences
Research Article
Forensic Identification of some Wild Animal Hair using Light and Scanning Electron Microscopy
Mayada Ragab Farag1*, Mervat Hassan Ghoniem1, Ali Heider Abou-Hadeed1, Kuldeep Dhama2
1Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Zagazig University, Egypt; 2Division of Pathology, Indian Veterinary Research Institute, Izatnagar, Bareilly, 243122, Uttar Pradesh, India.
Abstract | The present study was conducted to investigate some morphological and numerical features of hair samples of some wild animal species using light and scanning electron microscopy to make a key for their identification. Samples of hair were obtained from different body regions (dorsal neck, dorsum and flank) of American black bear (Urus americanus), Blue Nile monkey (Ceropithecus mitis), Barbary sheep (Amotracus lervia), Bactrian camel (Camelus bactrianus) and Llama (Lama glama). The measurements and indices used are the diameter of hair shaft at three positions (proximal, middle and distal parts of hair shaft), medulla and cuticular indices, as well as the scale count and the ratio of scale width to scale height (X/Y feret). The diameter of the proximal part of hair shaft showed very small variations in dorsal neck, dorsum or flank between the individuals of the same species and the same observed for the diameter of middle and distal parts, where the differences in the hair thickness among species were clearly observed by changing the part of hair and body region. The medullary and cuticular indices of individuals from the same species showed very small variations by changing the body region, but on the species level they showed significant differences. The animals of the same species are more likely to have similar scale patterns and count along the shaft of hair and even in the different parts of the body. On the other hand scale patterns and count showed great variations between different species along the hair shaft and also according to the body region. The species differences in the numerical features of cuticular scales were more frequently observed at the tip side than at the root. Barbary sheep has the largest X/Y feret value among species. In conclusion, the differences in the morphological and numerical features of hair used in this study could be of great value for species identification.
Keywords | Hair, Identification, SEM analysis, Wild animals
Editor | Muhammad Munir (PhD), Avian Viral Diseases Programme, The Pirbright Institute, Woking, Surrey, GU24 0NF, UK.
Received | July 13, 2015; Revised | August 18, 2015; Accepted | August 19, 2015; Published | September 22, 2015
*Correspondence | Mayada Ragab Farag, Zagazig University, Egypt; Email: [email protected]
Citation | Farag MF, Ghoniem MH, Abou-Hadeed AH, Dhama K (2015). Forensic identification of some wild animal hair using light and scanning electron microscopy. Adv. Anim. Vet. Sci. 3(10): 559-568.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.10.559.568
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Farag et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The mammalian hair fibers represent an interesting biological material which can be easily sampled, collected and transported as well as resist putrefaction and remain for very long periods of time so could provide long-term information (Nowak, 1998; Tridico et al. 2014). Moreover, in forensic casework the present hair samples are sometimes the only available evidence found at a crime scene (Brauner et al., 2001; Farah et al., 2014). Furthermore, the hair morphology is useful for the study of evolution and domestication of various mammals in zoology, morphology, phylogenetic, taxonomic, textile testing, archeological studies and forensic sciences (Meyer et al., 2002; Farag et al., 2015).
The macro and microscopic structure features are widely used for identification of hair and investigation of their role in adaptation of animals to life conditions. The former include the degree of hair cover the body with different coloration, shape, length and width, as well as position, arrangement in groups and direction of villi. The latter comprise the specific features of the hair shaft architectonics (ratio of development of three layers, cuticle, cortex and medulla; heterogeneity and pigmentation of cortical layer; cuticular pattern; shape of cuticular scales; and the shape, size, pigmentation and position of medullar cells and cavities between or inside them) (Chernova, 2002; Nadia, 2012; Monica et al., 2015).
Most studies on mammalian hair was done using plastic impressions of cuticular scales and direct observation of whole mounts using light microscopy (Brunner and Coman, 1974; Valente, 1983; Wallis ,1993; Oli, 1993; Taru and Backwell , 2013), and scanning electron microscopes (SEM) which provide new technology that allowed for greater magnification and resolution (Andy and Tillman, 2006; Aris and George, 2008).
This study aims at using light and scanning electron microscopy to obtain some features of hair samples from different body regions (dorsal neck, dorsum and flank) of some wild animals in trying to differentiate between them.
MATERIALS AND METHODS
The Tested Species
Samples of hair were obtained from five animal species from Giza Zoo, Giza, Egypt. All species used in this study were listed in Table 1.
Examination of Hair Samples using Light and Scanning Electron Microscopy (L.M. and SEM)
Hair samples were collected from each of five adult healthy males of selected species (Table 1).Ten hairs were collected from each of three different body regions, that is dorsal neck, dorsum and flank. All samples were morphologically and numerically examined in this study.
Whole Mounts
The samples were cleaned thoroughly in an ether-alcohol mixture (1:1) and were dried between two filter papers. The samples were placed on a clean microscope slides. Then the samples were mounted in Canada balsam with (refractive index: 1.53), and were examined under light microscope (VM 250) with magnification of 400X according to Oli (1993) then photographed using (SONY, DSC-S950) camera.
Scanning Electron Microscope
In order to examine the pattern of the cuticular scales and to calculate the scale counts per (100µm) unit length and the ratio of scale width to scale height (X/Y feret): untreated hairs from different types of animals mentioned in Table 1 were cleaned according to the procedure of Hess et al. (1985) which is as follows, hair samples were placed in small petri dishes with distilled water containing a drop of detergent (Baby Johnson) and sonicated for 5 min. Hairs were then washed in distilled water and sonicated for 5 minutes in absolute acetone. Hairs left to be air dried then mounted on SEM stubs. All the specimens were placed in a vacuum champer and gold coated (Edwards sputter coater S150 B) before being examined with a JEOL JSM- T100 Scanning Electron Microscope. The micrographs were taken at 20 KV and 1,000 X.
Table 1: The names and classification of animal’s source of hair samples
|
Common name |
Order |
Family |
Scientific name |
|
American black bear |
Carnivora |
Ursidae |
Urusamericanus |
|
Blue nile monkey |
Primates |
Ceropithecidae |
Ceropithecusmitis |
|
Barbary sheep |
Artiodactyla |
Bovidae |
Amotracuslervia |
|
Bacterian camel |
Artiodactyla |
Camelidae |
Camelusbactrianus |
|
Llama |
Artiodactyla |
Camelidae |
Lama glama |
Measurements and Indices used in this Study
The measurements and indices used were calculated according to Sato (2002) and Sato et al. (2006) as given below:
These features were measured at three positions of the hair shaft i.e. the proximal, middle and distal parts. These parts were objectively controlled as follows, the proximal part was at the proximal one third of the hair shaft (toward the tip of hair), the middle part was at the central part along the hair shaft, the distal part was at the maximum width point near the root of the hair, and the measurements obtained were adjusted to the actual size of the sample using ultra structure size calculator (SPI supplies, Divisions of structure probe, Inc. USA, units of measurements 1mm= 1000µm, 1µm= 10,000 Ao, 1 nm= 10 Ao).
Statistical Analysis
Data were statistically analysed using general linear models procedure adapted by SPSS for user’s guide with one-way ANOVA. The differences among means were determined using the student Newman keuls test. The Mean values and standard error (SE) were reported. Statements of statistical significance were based on P < 0.05.
Table 2: The measurements of the hair shaft (basal, middle and proximal parts) from dorsal neck, dorsum and flank regions of American black bear (Urus americanus)
|
Parameters |
Basal part |
Middle part |
Proximal part |
||||||
|
Dorsal neck region |
Dorsum region |
Flank region |
Dorsal neck region |
Dorsum region |
Flank region |
Dorsal neck region |
Dorsum region |
Flank region |
|
|
Diameter (µm) |
56.6± 0.66 a |
54.66± 2.6 a |
57.6± 0.66 a |
37.8± 0.16a |
38.0± 0.57 a |
38.6± 0.4 a |
31.1± 0.7a |
30.3± 0.3a |
31.3± 0.7a |
|
Medullary index |
36.7± 1.5b |
34.5± 0.28b |
41.0± 0.57a |
44.7± 0.3 a |
41.0± 0.57a |
41.3± 0.6 a |
37.9± 0.3a |
36.3± 0.88a |
36.7± 1.8a |
|
Cuticular index |
6.8± 0.16 b |
7.0± 0.00 b |
7.5± 0.00 a |
6.5± 0.11 a |
6.46± 0.21 a |
6.5± 0.24 a |
7.3± 0.20 a |
7.33± 0.18a |
7.30± 0.2a |
|
Cortical index |
31.0± 0.0 a |
31.0± 0.05 a |
31.3± 0.88a |
30.0± 0.0 a |
30.0± 1.00 a |
30.6± 1.2a |
30.8± 0.05a |
30.7± 0.08a |
30.3± 0.4a |
|
Scale counts per (100µm) |
25.0± 0.0 a |
24.3± 1.33 a |
24.3± 0.66 a |
20.0± 0.57 a |
20.3± 1.45 a |
20.0± 2.0 a |
12.0± 0.57a |
11.0± 0.57a |
12.3± 0.3a |
|
X/Y feret |
3.4± 0.08 a |
3.4± 0.03 a |
3.4± 0.06 a |
2.4± 0.05 a |
2.5± 0.00 a |
2.3± 0.24 a |
1.1± 0.03a |
1.10± 0.57 a |
1.2± 0.08 a |
Means within the same row in each item within each group carrying different superscripts are significantly different at (p< 0.05)
Table 3: The measurements of the hair shaft (basal, middle and proximal parts) from dorsal neck, dorsum, and flank regions of Blue Nile monkey (Ceropithecus mitis)
|
Parameters |
Basal part |
Middle part |
Proximal part |
||||||
|
Dorsal neck region |
Dorsum region |
Flank region |
Dorsal neck region |
Dorsum region |
Flank region |
Dorsal neck region |
Dorsum region |
Flank region |
|
|
Diameter (µm) |
45.83± 1.01a |
45.83± 0.44a |
45.00± 1.52a |
42.76± 0.14 a |
41.83± 1.16a |
41.96± 0.54 a |
31.00± 0.57a |
31.00± 1.15a |
29.00± 0.57a |
|
Medullary index |
49.86± 1.39a |
47.66± 1.85a |
49.00 ±1.52a |
48.16± 0.60 a |
48.33± 1.20a |
49.83± 0.16 a |
50.00± 0.57a |
49.66± 0.88a |
50.33± 0.33a |
|
Cuticular index |
3.10± 0.10a |
3.10± 0.05a |
3.20± 0.11a |
3.1± 0.57 a |
3.5± 0.11a |
3.4± 0.17 a |
4.00± 0.057a |
3.96± 0.08a |
4.00± 0.057a |
|
Cortical index |
32.00± 0.57a |
32.00± 1.55a |
32.66 ±1.76a |
32.00 ±1.00 a |
32.90± 0.73a |
33.00± 0.75a |
33.20± 0.05b |
32.80± 0.41b |
34.33± 0.16a |
|
Scale counts per (100µm) |
25.33± 0.33a |
25.00± 2.51a |
26.33 ±0.33a |
22.33± 1.20 a |
22.00± 1.00 a |
23.66± 0.88a |
18.33± 0.33 a |
19.33± 0.33b |
21.00± 0.00c |
|
X/Y feret |
3.50± .288 a |
3.60± 0.20 a |
4.00± 0.00 a |
3.00± 0.00 b |
3.13± 0.033 a |
3.10± 0.00a |
3.00± 0.00 a |
3.10± 0.00 a |
3.10± 0.05 a |
Means within the same raw in each item within each group carrying different superscripts are significantly different at (p< 0.05)
RESULTS AND DISCUSSION
Morphological examination of hair samples is the first step
in forensic hair examination. The main medico-legal concerns with hair examination include identification of the species origin, ascertainment of the hair’s provenance from the body and, finally, comparison of the hair sample from the victim to the hair sample from the crime scene (Zafarina and Panneerchelvam, 2009). It is well-known that based on morphological features some hairs and consequently some animal species can be discriminated without any trouble, that makes the morphological examination of hairs is an important method that can be used in forensic medicine (Sato, 2002; Sato et al., 2006).
Two of the features that make hair a good subject for establishing individual identity are its resistance to chemical decomposition and its ability to retain structural features over long period of time. Much of resistance and stability is attributed to the cuticle or outside covering of hair (Bernadette et al., 1996; Nowak et al., 1998).
Our investigation concerning the diameter of hair revealed that the diameter of the proximal part of hair shaft showed very small or no variations when compared in the three examined body regions (dorsal neck, dorsum and flank) between the individuals of the same species. There were also non-significant differences in the diameter of middle and distal part of hair shaft in the three body regions of the same species but with comparing the diameter of hairs on the species level the differences in the hair thickness among species were clearly observed by changing the part of hair and body region. Similar results were obtained by
Table 4: The measurements of the hair shaft (basal, middle and proximal parts) from dorsal neck, dorsum and flank regions of Barbary sheep (Amotracus lervia)
|
Parameters |
Basal part |
Middle part |
Proximal part |
||||||
|
Dorsal neck region |
Dorsum region |
Flank region |
Dorsal neck region |
Dorsum region |
Flank region |
Dorsal neck region |
Dorsum region |
Flank region |
|
|
Diameter (µm) |
126.66 ±1.20 a |
126.00± 1.52 a |
127.00 ± 2.08 a |
95.00 ±0.00 a |
95.33 ±1.85a |
95.66 ± 1.86 a |
67.50 ±0.28 b |
67.00± 0.00 b |
68.33± 0.33 a |
|
Medullary index |
86.13± 0.88 a |
86.23± 0.62 a |
87.16± 0.44 a |
86.5± 0.10 a |
86.4± 0.057 a |
86.2± 0.43 a |
81.166± 0.16 a |
81.66± 0.33 a |
82.00± 0.57 a |
|
Cuticular index |
3.03± 0.03 a |
2.93± 0.03 a |
3.06± 0.14 a |
3.03± 0.033 b |
3.10± 0.00 b |
3.23± 0.033 a |
3.60± 0.57 a |
3.50± 0.10 a |
3.63± 0.145 a |
|
Cortical index |
8.06± 0.033 a |
8.20± 0.05 a |
8.20± 0.05 a |
7.7± 0.058 a |
7.5± 0.145 a |
7.4± 0.06 a |
7.30± 0.05 b |
7.40± 0.05 b |
7.60± 0.00 a |
|
Scale counts per (100µm) |
143.33± 6.66 a |
141.33± 1.20 a |
143.33 ±3.33 a |
88.33± 3.33 a |
88.34± 4.40 a |
86.66± 3.33 a |
90.00± 2.88 a |
91.66± 3.33 a |
92.33± 6.17 a |
|
X/Y feret |
7.00± 0.00 a |
7.00± 0.01 a |
7.00± 0.152 a |
5.00± 0.057 a |
5.00± 0.00 a |
4.90± 0.25 a |
5.33± 0.71 a |
6.03± 0.03 a |
6.03± 0.03 a |
Means within the same raw in each item within each group carrying different superscripts are significantly different at (p<05).
Table 5: The measurements of the hair shaft (basal, middle and proximal parts) from dorsal neck, dorsum and flank regions of Llama (Lama glama)
|
Parameters |
Basal part |
Middle part |
Proximal part |
||||||
|
Dorsal neck region |
Dorsum region |
Flank region |
Dorsal neck region |
Dorsum region |
Flank region |
Dorsal neck region |
Dorsum region |
Flank region |
|
|
Diameter (µm) |
44.66± 1.33a |
46.00± 0.57a |
47.13± 0.18a |
37.66±0.33a |
37.66± 0.16a |
38.00± 0.57 a |
34.00± 0.57 a |
34.33± 0.06 a |
34.00± 0.57 a |
|
Medullary index |
34.16± 0.44a |
33.33± 0.28a |
34.16± 0.44a |
33.96± 0.31a |
33.33± 0.88a |
33.36± 0.31 a |
34.90± 0.49 a |
34.00± 0.57 a |
35.10± 0.01a |
|
Cuticular index |
3.00± 0.00 a |
3.00± 0.05a |
3.03± 0.12a |
3.13±0.08a |
3.13± 0.06a |
3.20± 0.05a |
3.26± 0.12a |
3.23± 0.12a |
3.26± 0.06 a |
|
Cortical index |
42.36± 0.31a |
42.63± 0.68a |
42.00± 1.73a |
32.00±1.00a |
42.50± 0.76a |
42.10± 0.44a |
42.30± 0.25a |
42.53± 0.26a |
42.13± 0.40 a |
|
Scale counts per (100µm) |
13.00± 0.57a |
12.66± 1.20a |
12.66± 1.45a |
23.33± 2.02a |
23.33± 0.08a |
23.66± 1.33a |
35.00± 1.15a |
33.33± 0.88a |
33.00± 1.52 a |
|
X/Y feret |
1.56±0.03a |
4.80± 3.35a |
1.50± 0.10a |
2.03± 0.03a |
2.10± 0.05a |
2.00± 0.00 a |
1.26± 0.033 a |
1.23± 0.066 a |
1.30± 0.00 a |
Means within the same raw in each item within each group carrying different superscripts are significantly different at (p< 0.05)
(Sato et al., 2006) who found that numerical features such as hair diameter can clearly be used to differentiate between hairs of dog and cat. Our results also in agreement with the results of Aris and George (2008) who studied the morphology of hairs of Capra prisca (goat) and the sheep wool and found that the diameter of hairs of goat and the diameter of wool varied greatly, they concluded that hair diameter could be useful for hair identification of mammalian species. Moreover, Jones et al. (2001) stated that diameter of hair shaft between species can vary from 10-250 µm and are influenced by the metabolic and nutritional status of the animal, which may play indirect role in the variability of hair diameter observed among different species used in our study.
This study also revealed that the medullary index of individuals from the same species showed very small or no variations by changing the body region but comparing medullary index on the species level showed significant differences and was less than 1/3 in human hairs while that of animals was greater than 1/3 except for bactrian camel which have medullary index less than 1/3. These results are in agreement with Gaudette (1999) and Deedrick and Koch (2004) who stated that the medulla of hairs also valuable for species identification, animal medullary index is greater than human’s. Moreover the differences in medullary index and patterns observed between different species may be explained by Chernova (2003) who stated that the different structure of the medulla could be returned to structural and functional adaptation because the medulla is related to the thermal insulation of hair coat of different animals.
Table 6: The measurements of the hair shaft (basal, middle and proximal parts) from dorsal neck, dorsum and flank regions of Bacterian camel (Camelus bactrianus)
|
Parameters |
Basal part |
Middle part |
Proximal part |
||||||
|
Dorsal neck region |
Dorsum region |
Flank region |
Dorsal neck region |
Dorsum region |
Flank region |
Dorsal neck region |
Dorsum region |
Flank region |
|
|
Diameter (µm) |
47.33± 1.20a |
45.66± 0.88a |
47.66± 0.57a |
43.16± 0.44b |
42.73± 0.14b |
44.23± 0.14a |
33.00±0.57a |
31.33± 0.88a |
31.00± 1.15a |
|
Medullary index |
16.66± 0.44b |
17.70± 0.05a |
17.46± 0.16b |
16.63± 0.49a |
16.73± 0.27a |
16.86± 0.28a |
16.50±0.28a |
16.66± 0.44a |
16.80± 0.40a |
|
Cuticular index |
2.30± 0.11a |
2.30± 0.17a |
2.30± 0.05a |
2.80± 0.05a |
2.86± 0.03a |
2.56± 0.17a |
2.70± 0.25a |
2.60± 0.15a |
2.66± 0.14a |
|
Cortical index |
33.00± 0.57a |
33.83± 0.60a |
34.16± 0.44a |
34.83± 0.16a |
34.70± 0.30a |
34.16± 0.60a |
33.33± 0.33a |
33.50± 0.76 a |
34.00± 1.15a |
|
Scale counts per (100µm) |
13.00± 0.00a |
12.33 ± 0.88a |
12.00± 1.00a |
11.66± 0.33a |
11.66± 0.66a |
11.00± 1.52a |
9.00± 0.57a |
8.33± 0.66a |
7.66± 0.66a |
|
X/Y feret |
1.33± 0.08a |
1.33± 0.16a |
1.26± 0.13a |
1.40± 0.05a |
1.36± 0.15a |
1.40± 0.12a |
1.16±0.03a |
1.20± 0.00 a |
1.20± 0.05a |
Means within the same raw in each item within each group carrying different superscripts are significantly different at (p< 0.05)
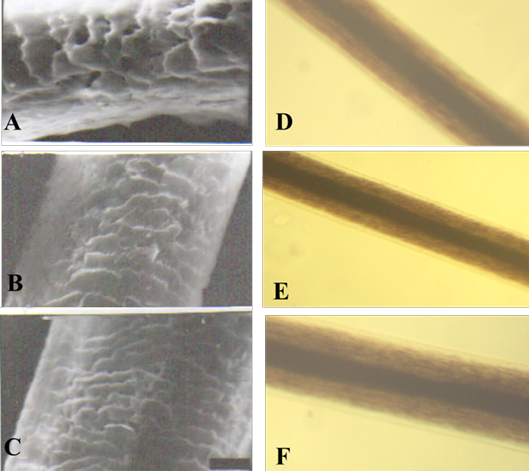
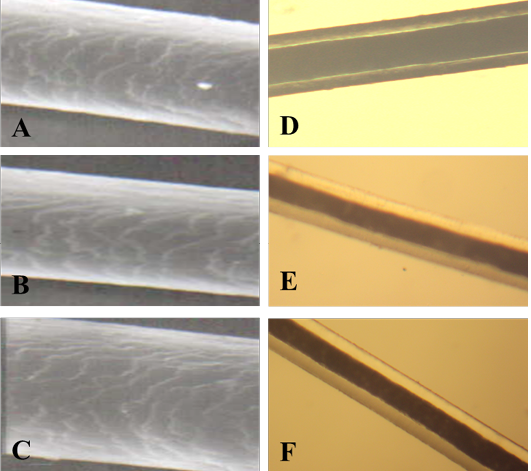
Figure 1: SEM of the hair shaft of Urus americanus
A: proximal part, B: middle part and C: distal part; note the thickness of hair shaft, note also the overlapped short and wide imbricated scales of equal hastate with saw margin and without long free blade (X1000). The other side shows the three morphological regions of hair (the cuticle, the medulla and the cortex) along the hair shaft of Urus americanus (D: proximal part, E: middle part and F: basal part) under light microscope (X400)
Regarding to the results of cuticular index our study revealed that there were no or very small differences in the cuticular index among individuals of the same species by examining the different parts of hair at different body region, meanwhile,there was a great difference in cuticular index among different species and this is in agreement with that mentioned by Jones et al. (2001) who reported that cuticle thickness varies markedly between species, for example the cuticle of fine merino wool fibers is normally one cell thick whereas in human hair and pig bristle the cuticle
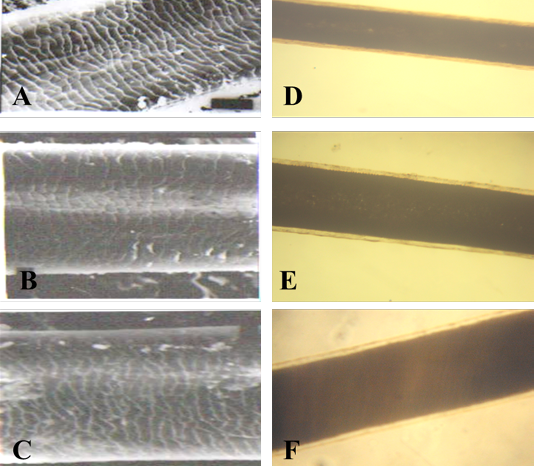
A: proximal part, B: middle part and C: distal part; note the thickness of hair shaft, note also the overlapped moderate length and wide imbricated scales of equal hastate with saw margin and without long free blade (X1000). The other side shows the three morphological regions of hair (the cuticle, the medulla and the cortex) along the hair shaft (D: proximal part, E: middle part and F: basal part) of Ceropithecus mitis under light microscope (X400)
may range from 10 cells to 30 cell layers respectively and the author related this to the unique characteristics of hair cuticle cells which give mammalian hair its surface properties, these cells are important in a variety of applications ranging from textile processing to protection of fiber component from environmental damage.
The examination of hair shaft with the aid of SEM provided useful information of the hair morphology of studied species concerning scale counts and the ratio of scale
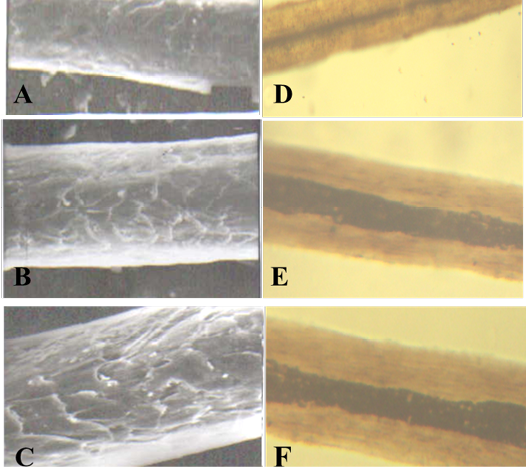
A: proximal part, B: middle part and C: distal part; note the thickness of hair shaft, note also the overlapped short and wide imbricated scales of equal hastate with smooth margin and without long free blade (X1000). The other side shows the three morphological regions of hair (the cuticle, medulla and the cortex) along the hair shaft of Amotracus lervia (D: proximal part, E: middle part and F: basal part) under light microscope (X400)
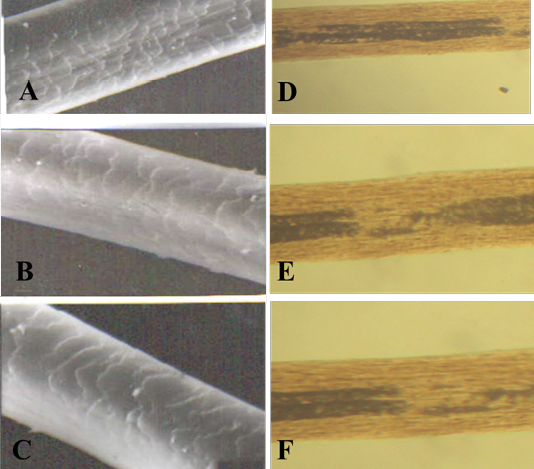
Figure 4: SEM of the hair shaft of Lama
A: proximal part, B: middle part and C: distal part; note the thickness of hair shaft, note also the overlapped moderate length and moderate width imbricated scales of equal hastate with saw margin and without long free blade (X1000). The other side shows the three morphological regions of hair (the cuticle, the medulla and the cortex) along the hair shaft of Lama glama (D: proximal part, E: middle part and F: basal part) under light microscope (X400)
Table 7: The measurements and indices of the basal part of hair shaft of different body regions (dorsal neck, dorsum and flank) of the studied animals
|
Animal species |
American black bear |
Blue nile monkey |
Barbary sheep |
Lama |
Bacterian camel |
|
Body regions |
|||||
|
Dorsal neck region |
|||||
|
Diameter (µm) |
31.16± 0.72c |
31.00± 0.57 c |
67.50±0.28a |
34.00±0.57b |
33.00± 0.57b |
|
Medullary index |
37.96± 0.29c |
50.00± 0.57b |
81.16± 0.16a |
34.90± 0.49d |
16.5± 0.28e |
|
Cuticular index |
7.33± 0.20a |
4.00± 0.057b |
3.60± 0.057bc |
3.26± 0.12c |
2.70± 0.5d |
|
Cortical index |
30.80± 0.05c |
33.20± 0.05b |
7.30± 0.05d |
42.30± 0.25a |
33.33± 0.33b |
|
Scale counts per (100µm) |
12.00± 0.57d |
18.33 ± 0.33c |
90.00± 2.88a |
35.00± 1.15b |
9.00± 0.57d |
|
X/Y feret |
1.06± 0.03d |
2.86± 0.08b |
6.00± 0.05a |
1.26± 0.03c |
1.16± 0.03cd |
|
Dorsum region |
|||||
|
Diameter (µm) |
30.33± 0.33c |
31.00± 1.10c |
67.00± 0.00a |
34.33± 0.66b |
31.33± 0.88c |
|
Medullary index |
36.33± 0.88c |
49.66± 0.88b |
81.66± 0.33a |
34.00± 0.57d |
16.56± 0.34e |
|
Cuticular index |
7.33± 0.18a |
3.96± 0.08b |
3.50± 0.10c |
3.23± 0.12c |
2.60± 0.15d |
|
Cortical index |
30.76± 0.08c |
33.10± 0.15b |
7.40± 0.05d |
42.53± 0.26a |
33.50± 0.76b |
|
Scale counts per (100µm) |
11.00± 0.57d |
19.33± 0.33c |
91.66± 3.33a |
33.33± 0.88b |
8.33±0.66d |
|
X/Y feret |
1.10± .05c |
2.90± 0.20b |
6.03± 0.03a |
1.23± 0.06c |
1.20± 0.00c |
|
Flank region |
|||||
|
Diameter (µm) |
31.33± 0.66c |
29.00± 0.57c |
68.33 ± 0.33a |
34.00 ± 0.57b |
31.00± 1.15c |
|
Medullary index |
36.66± 1.85c |
50.33± 0.33 b |
82.00± 0.57a |
35.16 ± 0.16c |
16.80 ± 0.40d |
|
Cuticular index |
7.30± 0.15a |
4.00± 0.05b |
3.63± 0.14bc |
3.26± 0.06c |
2.66 ± 0.14 d |
|
Cortical index |
30.30± 0.35c |
34.26± 0.14b |
7.60± 0.00d |
42.13 ± 0.40a |
34.00 ± 1.15b |
|
Scale counts per (100µm) |
12.33± 0.33cd |
21.33 ± 0.33c |
92.33 ± 6.17a |
33.00± 1.52b |
7.66 ± 066d |
|
X/Y feret |
1.16± 0.08c |
2.93± 0.03b |
6.03± 0.03a |
1.30± 0.00c |
1.20± 0.05c |
Means within the same raw in each item within each group carrying different superscripts are significantly different at (p< 0.05)
Table 8: The measurements and indices of the middle part of hair shaft of different body regions (dorsal neck, dorsum and flank) of the studied animals
|
Animal species |
American black bear |
Blue nile monkey |
Barbary sheep |
Lama |
Bacterian camel |
|
Body regions |
|||||
|
Dorsal neck region |
|||||
|
Diameter (µm) |
37.83± 0.16c |
42.76± 0.14b |
95.00± 0.00a |
37.66 ± 0.33c |
43.16± 0.44b |
|
Medullary index |
40.66± 0.33c |
48.16± 0.60b |
86.50± 0.01a |
33.96± 0.31d |
16.63± 0.49e |
|
Cuticular index |
6.50± 0.11a |
3.10± 0.05b |
3.03 ± 0.03 bc |
3.13± 0.08b |
2.80± 0.05c |
|
Cortical index |
30.00± 0.00d |
32.00± 1.00c |
7.70± 0.05e |
42.66± 0.33a |
34.83± 0.16b |
|
Scale counts per (100µm) |
20.00 ± 0.57b |
22.33 ± 1.20b |
88.33 ± 3.30a |
23.33± 2.00b |
11.66± 0.33c |
|
X/Y feret |
2.40± 0.05c |
3.00± 0.00b |
5.00± 0.05a |
2.03± 0.03d |
1.40± 0.05e |
|
Dorsum region |
|||||
|
Diameter (µm) |
38.00± 0.57c |
41.83± 1.16b |
95.33± 1.85a |
37.66± 0.16c |
42.66± 0.20b |
|
Medullary index |
41.00± 0.57c |
48.33± 1.20b |
86.40± 0.05a |
33.33± 0.88d |
16.73± 0.27e |
|
Cuticular index |
6.46± 0.21a |
3.50± 0.11b |
3.10± 0.00c |
3.13 ± 0.06c |
2.86± 0.03c |
|
Cortical index |
30.00± 1.00c |
32.90± 0.73b |
7.56± 0.14d |
42.50± 0.76a |
34.70± 0.30b |
|
Scale counts per (100µm) |
20.00± 2.00b |
22.00± 1.00b |
88.33 ± 4.40a |
23.33± 0.88b |
11.66± 0.66c |
|
X/Y feret |
2.50± 0.00c |
3.13± 0.03b |
5.00± 0.00a |
2.10± 0.05 d |
1.36± 0.12e |
|
Flank region |
|||||
|
Diameter (µm) |
38.50± 0.50c |
41.96± 0.54b |
95.66± 1.85a |
38.00± 0.57c |
44.23± 0.14b |
|
Medullary index |
41.33± 0.66c |
49.83± 0.16b |
86.20±0.43a |
33.36± 0.31d |
16.86± 0.28e |
|
Cuticular index |
6.56± 0.24a |
3.46± 0.17b |
3.23± 0.03b |
3.20± 0.05b |
2.56± 0.17c |
|
Cortical index |
30.66± 1.20c |
33.00 ± 0.57b |
7.43±0.06d |
42.16± 0.44a |
34.16± 0.60b |
|
Scale counts per (100µm) |
20.00± 2.00b |
23.66 ± 0.88b |
86.66± 3.33a |
23.66± 1.33b |
11.00± 1.52c |
|
X/Y feret |
2.00± 0.52c |
3.10± 0.00b |
5.03 ± 0.12a |
2.00± 0.00c |
1.40± 0.15c |
Means within the same raw in each item within each group carrying different superscripts are significantly different at (p< 0.05)
A: proximal part, B: middle part and C: distal part; note the thickness of hair shaft, note also the overlapped moderate length and moderate width imbricated scales of equal hastate with saw margin and without long free blade (X1000). The other side shows the three morphological regions of hair (the cuticle, the medulla and the cortex) along the hair shaft of Camelus bactrianus (D: proximal part, E: middle part and F: basal part) under light microscope (X400)
width to scale height. Bower and Curry (1983) reported that scale patterns provided some of the most diagnostic characters for identifying hair samples.
Regarding to the results of scale patterns we found that the animals of the same species are more likely to have similar scale patterns along the shaft of hair and even in the different parts of the body. On the other hand scale patterns show great variations between different species as shown in Figures 1, 2, 3, 4 and 5 which appeared as moderate length (short and wide) and sometimes have smooth margin or saw margin according to the species of animal. This is in agreement with the observation of Hess et al. (1985) who found that the surface scale patterns of Tayasuidae and Suidae family did not significantly differ when observed with scanning electron microscope. Inagaki and Tsukahara (1993) and Bakuneeta et al. (1993) used scale patters to identify chimpanzee hair.
The study also revealed no significant difference in the scale counts of the proximal part, middle and basal parts of hair shaft when compared in the three body regions of the animals of the same species (Tables 2, 3, 4, 5 and 6). Meanwhile, comparing the scale counts in the different species
Table 9: The measurements and indices in the proximal part of hair shaft at different body regions (dorsal neck, dorsum and flank) of the studied animals
|
Animal species |
American black bear |
Blue nile monkey |
Barbary sheep |
Lama |
Bacterian camel |
|
Body regions |
|||||
|
Dorsal neck region |
|||||
|
Diameter (µm) |
31.16± 0.72c |
31.00± 0.57 c |
67.50±0.28a |
34.00±0.57b |
33.00± 0.57b |
|
Medullary index |
37.96± 0.29c |
50.00± 0.57b |
81.16± 0.16a |
34.90± 0.49d |
16.5± 0.28e |
|
Cuticular index |
7.33± 0.20a |
4.00± 0.057b |
3.60± 0.057bc |
3.26± 0.12c |
2.70± 0.5d |
|
Cortical index |
30.80± 0.05c |
33.20± 0.05b |
7.30± 0.05d |
42.30± 0.25a |
33.33± 0.33b |
|
Scale counts per (100µm) |
12.00± 0.57d |
18.33 ± 0.33c |
90.00± 2.88a |
35.00± 1.15b |
9.00± 0.57d |
|
X/Y feret |
1.06± 0.03d |
2.86± 0.08b |
6.00± 0.05a |
1.26± 0.03c |
1.16± 0.03cd |
|
Dorsum region |
|||||
|
Diameter (µm) |
30.33± 0.33c |
31.00± 1.10c |
67.00± 0.00a |
34.33± 0.66b |
31.33± 0.88c |
|
Medullary index |
36.33± 0.88c |
49.66± 0.88b |
81.66± 0.33a |
34.00± 0.57d |
16.56± 0.34e |
|
Cuticular index |
7.33± 0.18a |
3.96± 0.08b |
3.50± 0.10c |
3.23± 0.12c |
2.60± 0.15d |
|
Cortical index |
30.76± 0.08c |
33.10± 0.15b |
7.40± 0.05d |
42.53± 0.26a |
33.50± 0.76b |
|
Scale counts per (100µm) |
11.00± 0.57d |
19.33± 0.33c |
91.66± 3.33a |
33.33± 0.88b |
8.33±0.66d |
|
X/Y feret |
1.10± .05c |
2.90± 0.20b |
6.03± 0.03a |
1.23± 0.06c |
1.20± 0.00c |
|
Flank region |
|||||
|
Diameter (µm) |
31.33± 0.66c |
29.00± 0.57c |
68.33 ± 0.33a |
34.00 ± 0.57b |
31.00± 1.15c |
|
Medullary index |
36.66± 1.85c |
50.33± 0.33 b |
82.00± 0.57a |
35.16 ± 0.16c |
16.80 ± 0.40d |
|
Cuticular index |
7.30± 0.15a |
4.00± 0.05b |
3.63± 0.14bc |
3.26± 0.06c |
2.66 ± 0.14 d |
|
Cortical index |
30.30± 0.35c |
34.26± 0.14b |
7.60± 0.00d |
42.13 ± 0.40a |
34.00 ± 1.15b |
|
Scale counts per (100µm) |
12.33± 0.33cd |
21.33 ± 0.33c |
92.33 ± 6.17a |
33.00± 1.52b |
7.66 ± 066d |
|
X/Y feret |
1.16± 0.08c |
2.93± 0.03b |
6.03± 0.03a |
1.30± 0.00c |
1.20± 0.05c |
Means within the same raw in each item within each group carrying different superscripts are significantly different at (p< 0.05)
revealed significant differences along the hair shaft and also according to the body region (Tables 7, 8 and 9). The species differences in the numerical features of cuticular scales were more frequently observed at the tip side than at the root and this disagreed with Sato et al. (2006) who suggested that scale counts at the distal part of hair shaft are important for the species discrimination when numerical features are used.
Tonin et al. (2002) and Aris and George (2008) pointed out that the fiber diameter and scale pattern type as well as the rate of growth in fiber length and the distance between scale ridges are related to each other, they suggested that the thicker hairs showed higher density of scales, the results of our study confirmed the latter statement where barbary sheep have the greater diameter of hair shaft and higher density of scales and at the same time has the largest X/Y value among studied species and this might be due to the habitat of animal to accommodate itself during cold periods to increase the degree of insulation as the ratio of scale width to height could reflect an effective function for the cuticle scales in supporting (i.e. holding up) the hairs, these scales form a segmented tube that can support the cortex when hairs erected during cold periods in order to improve insulation. The hair cuticle cells have an extremely keratinized outer core which corroborates this idea and they are very resistant to environment (Jones and Rivett, 1997; Dobb et al., 1996; Areida et al., 2006).
The ratio of scale width to scale height clearly indicates that the species specific shape and size of hair cuticle scales in mammals may be of specific value for biological interpretation with regard to hair coat structure and function (Meyer et al., 2002; Areida et al., 2006).
From the observations of the present study we agreed with (Sato et al., 2006) who found that the morphological differences that are useful for species identification of animal hairs can be observed between animals classified at taxonomic position at relatively long distances from one another. Moreover animal hair morphologies may be influenced by habitat and body size, for example morphological differences in animal hairs were clearly observed between an aquatic animal and land animal and between a large sized animal and a small animal. On the other hand hair morphologies are similar between animals belonging to the same family and genus such as a dog and wolf (canidae canis) and are relatively similar between animals bearing some resemblance in terms of body size.
Conclusion
The measurements and indices used in the present study could provide useful information about hair from each studied species that could be of great value in their identification.
ACKNOWLEDGEMENT
All the authors of the manuscript thank and acknowledge their respective Universities and Institutes.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR’S CONTRIBUTION
Mayada Ragab Farag and Mervat Hassan Ghoniem carried out the experiment trial, performed the statistics and drafted the manuscript as well as revised the manuscript. Ali Heider Abou-Hadeed conceived the study, and participated in its design and coordination. Kuldeep Dhama reviewed and revised the manuscript. All authors read and approved the final manuscript.
REFERENCES