Advances in Animal and Veterinary Sciences
Research Article
Impact of Stevioside Supplementation as Feed Additive in Finisher Broiler Diets on Growth Performance, Carcass Traits, Meat Quality, Selected Biochemical Parameters, and Caecum Microflora
Ayman Moawed Khalifah1*, Amina Shaban El-Saadany2, Mohamed Ibrahem Hassan1, Walaa Abdelraouf Kashyout1, Waleed Mostafa Dosoky3
1Livestock Research Department, Arid Lands Cultivation Research Institute, City of Scientific Research and Technological Applications (SRTA-City), New Borg El Arab, Egypt; 2Animal Production Research Institute, Agriculture Research Center, Egypt; 3Animal and Fish Production Department, Faculty of Agriculture (Saba Basha), Alexandria University- Egypt.
Abstract | Stevioside (STE) is the major ingredient of Stevia rebaudiana Bertoni leaves, plant from South America used to manufacture natural sweeteners. Stevia is used as a sweetener with no calories; evidence demonstrates that it has non-toxic effects on human verdure and is used in food production. The experiment was conducted to estimate the action of STE supplementation in finisher broiler diets. A total of 280 COBB 500 (19 d- old) male broilers were allotted to 4 experimental treatments (7 replicates per treatment). Treatments were: 1) control diet, 2) 1.5 g STE/kg feed (S1.5); 3) 3 g STE kg/feed (S3) and 4) 6 g STE/ kg feed (S6). Results showed that the growth performance (GP) traits were affected positively by STE supplementation (p< 0.01) compared to control. All carcass traits were not affected by STE supplementation except abdominal fat percentage, which decreased compared to control (P ≤ 0.05) as affected by STE supplementation. STE supplementation did not affect Meat quality, but it increases the shear-value (p< 0.01) of breast meat. A linear increase was shown in biochemical parameters: total protein, globulin, immunoglobulin A (IgA), and immunoglobulin G (IgG) concentration as affected by STE addition; inversely, a linear decrease has occurred in glucose concentration. Likewise, dietary STE supplementation improves the concentricity of lactobacillus and suppresses the concentricity of E-coli and salmonella. Results referred that the addition of STE with different levels in finisher broiler diets should enhance growth performance, breast meat quality, some blood biochemical parameters, and caecum microorganisms of broiler chicks. Hence, it may be used as a feed additive in finisher broiler diets.
Keywords | Stevioside, Broiler, Growth Performance, Meat Quality, Caecum Microflora
Received | August 29, 2021; Accepted | October 04, 2021; Published | November 01, 2021
*Correspondence | Ayman Moawed Khalifah, Livestock Research Department, Arid Lands Cultivation Research Institute, City of Scientific Research and Technological Applications (SRTA-City), New Borg El Arab, Egypt; Email: [email protected]
Citation | Khalifa AM, El-Saadany AS, Hassan MI, Kashyout WA, Dosoky WM (2021). Impact of stevioside supplementation as feed additive in finisher broiler diets on growth performance, carcass traits, meat quality, selected biochemical parameters, and caecum microflora. Adv. Anim. Vet. Sci. 9(12): 2168-2175.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.12.2168.2175
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Khalifah et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
With today’s tendencies oriented at bettering food and feed security, diminishing environmental pollution and general health hazards, it is necessary to find paths to reduce artificial components in our food and increase the natural products.
STE is a natural sweetener segregated from the leaves of Stevia rebaudiana Bertoni plant which is native from northeastern Paraguay, and it is reach to 300 times sweeter than sucrose (Alaam, 2007). The mainfine material for the making of STE is the leaves of stevia plant. The plant’s stem contains low rates of glycosides that are taken away during harvest to decrease the price of processing (Brandle et al.,, 1992).
STE was characterized by heat-stable, well-tolerated low pH values (Jana et al.,, 2013). The glycemic index is zero, thus it is a sweetener with no caloric value (Atteh et al., 2008; Puri et al., 2011); aside from the sweet content, it is due to its secondary plant components (phytochemicals). Stevia has antihyperglycemic, anti-inflammatory, diuretic, antibacterial, and immune-modulating effects and has proven non-toxic effect on human health when compared to synthetic sweeteners which determined by low caloric characteristics and a large percentage of carcinogenic compounds (Puri and Sharma 2011; Wang et al., 2015).
Nowadays high-potency sweeteners have been used in animal feed (Moran et al., 2014; Ma et al., 2017), because of its high sweetness content and low-calories. A few sweeteners are broadly utilized to promote the tastefulness of the animal diets (Figueroa et al., 2019) and extend biological assignments in the gut (Meyer-Gerspach et al., 2018; Hunter et al., 2019).
Nevertheless, data are lacking about the impact of sweeteners supplementation on broiler chicks. Some studies focused on the physiological connection between sweeteners content in chickens and the gastrointestinal tract (Kimmich et al., 1989). Atteh et al. (2008) suggest that dietary STE addition might affect GP in broiler chicks. Still, the results conflicted with Wu et al. (2019) and Daneshyar (2012), who reported that dietary STE does not affect GP, limited examination has estimated the action of dietary STE on lipid metabolism and glucose in broilers. Atteh et al. (2008) and Wu et al. (2019) stated that dietary STE addition in diets has decreased concentration of blood glucose and increased abdominal fat content in broilers. So, understanding the physiological and biological functions of sweeteners could help explore new feed additives for broiler chickens.
So, this study aimed to conduct the action of STE at finisher diets on broiler growth performance, carcass traits, meat quality, some biochemical parameters, and bacteria count for caecum.
Materials and Methods
This study was performed at the Fish and Animal Production Department, Faculty of Agriculture (Saba Basha), Alexandria University.
Experimental design and diets
The Institutional Animal Ethics Committee (Alexandria University) confirmed the field experiment under the number AU:19/21/04/22/3/17.
A total of 280 male 1d-old chicks COBB 500 received from Ismailia/Misr Company for poultry production and were fed a starter diet till 18 days of age. Then at 19 days, birds were divided to 4 experimental diets according to the initial body weight at the finisher period. There were seven replicate floor pens for each treatment, with ten birds pere pen. Treatments were: (1) control, (2) 1.5g/kg feed supplemental STE (S1.5), (3) 3 g/kg feed supplemental STE (S3), and (4) 6 g/kg feed supplemental STE (S6). All chicks were fed the finisher diets through the experimental period (19-35 days). The composing of the finisher diet was shown in Table 1. Diets were formed to meet or surpass broilers nutritional requirements reported by the NRC (1994).
Chicks were kept in a cleaned and fumigated ground floor under similar managerial conditions. Artificial lighting was provided 24 hours during experiment period. A gas heater was used to provide the chicks with the heat needed for brooding. An ambient temperature program was maintained at 27 °C from age 19 to 23 days decreased to 25 °C from 24 to 28 days and then decreased to 23 °C till the end of experiment. Feed and water were offered ad-libitum all over the experiment.
Determination of growth performance
Body weight (BW) and feed intake (FI) were fixed at the initial and end of the finisher period. Body weight gain (BWG) and feed conversion ratio (FCR) were calculated from these data. Determination of FCR was at the rate between feed intake and body weight gain.
Determination of carcass traits
Seven birds from each treatment were randomly chosen at 35 days old. Assigned birds fasted overnight. Broilers were hung in steel shackles by their feet by hand, and then they were slaughtered by cutting the jugular veins of the neck according to the Islamic religion instruction with a sharp knife. When complete bleeding was achieved, scalded, de-feathered, and manually eviscerated; Carcasses, breast, thigh, fillet, tender, thigh meat, abdominal fat, and wings were calculated as a percentage relative to live body weight. All carcasses stored in ice in metal containers after harvest, and later transferred to rubber containers for further analysis.
Determination of meat quality.
Breast Samples: Breast samples (7 samples for each treatment) were then individually vacuum-packaged and frozen
Table 1: Ingredient composition and calculated analysis of the basal diet for broiler chicks.
| Ingredients % | Finisher diet |
| Corn (yellow) | 60.88 |
| Soybean, (48% CP) | 33.30 |
| Soy oil | 2.90 |
| Limestone | 1.75 |
| Di- calcium phosphate | 0.20 |
| Common salt | 0.45 |
|
Vitamin premix1 |
0.10 |
|
Mineral premix2 |
0.20 |
| DL-methionine, (98%) | 0.15 |
|
Mixed enzymes3 |
0.030 |
|
Phytase4 |
0.005 |
|
Coccidiostat5 |
0.020 |
|
Anti-mycotoxin6 |
0.010 |
|
Probiotic7 |
0.006 |
| Total | 100 |
| ME, (kcal/kg) | 3100 |
|
Crude protein, (%) |
21 |
| Fat, (%) | 2.75 |
| Fiber, (%) | 2.45 |
| Calcium, (%) | 0.77 |
| Available Phosphorus, (%) | 0.38 |
| Total Lysine, (%) | 1.05 |
| Methionine, (%) | 0.45 |
| Cysteine, (%) | 0.28 |
| Meti+cyst, (%) | 0.73 |
| Arginine, (%) | 1.07 |
1Vitamin premix provides per diet: Vit. A; 12000000 IU, Vit. E: 400000 mg, Vit. Bl: 2000 mg. Vit. B2: 160000 mg, Vit.B6: 5000 mg, Vit, B12: 12 mg, Niacin: 45000 mg, Pantothenic acid: 12000 mg, Vit. K: 3000 mg, Vit. D3; 3000000 IU, Biotin: 70mg and Folic acid: 2000mg.
2 trace mineral premix provides per diet: Choline: 3600000 mg, Copper: 10000 mg, Iodine: 1000 mg, Iron: 30000 mg, Manganese: 100000 mg, Zinc: 600000 mg, and selenium: 400 mg, cobalt: 100 mg.
3 Combo® Enzyme Blend consists of: Cellulase 76,000 CU units/kg, Fungal amylase 31,000 SKB units/kg, Fungal protease 1,000,000 HUT units/kg, Neutral protease 200,000 PC units/kg, Alkaline protease 1.1 Anson units/kg, Xylanase 21,000 XU units/kg, Beta-glucanase 21,000 BG units/kg, Hemicellulase 21,000 HCU units/kg and Lipase 76,000 FIP units/kg.
4 Axtra® PHY 10000 TPT, 6-phytase 10000 FTU/g
5 Diclazuril 500 mg, Atozuril® (ATco pharma).
6Mycofix® Select 3, deactivate of mycotoxins
7 Enviva® Pro 202 GT, Bacillus subtilis 2.5E CFU/gm
(-23 oC) until determinations could be performed.
Cooking Loss: Frozen samples of breast fillets were melted at 2°C for 24 hours. The melted breast fillets samples were weighed and arranged on wire oven racks, cooked in a preheated convection oven (177 oC) until the desired internal temperature was reached. Breast fillets were cooked at77 oC, then ejected from the oven and let to cool to an internal temperature of 24 oC, and reweighed. Cooking loss (%) was supposed as a variance between the raw and cooked fillet weight by raw fillet weight x 100 (Saenmahayaket al., 2012).
Shear-Value: Using the approach outlined by Sams et al. (1990) shear values were determined using a TA.HDi Heavy Duty texture analyzer (Stable Micro Systems Ltd., Godalming, Surrey, UK) fitted with an Allo-Kramer shear cell. One meat sample (about 2 × 4 × 1 cm) was sliced parallel to the muscle fibre direction, weighed, and sheared at a right angle to the fibres using a 250-kg weight.The shear values were expressed in kilograms per gram of sample.
Thigh samples: Seven broiler thigh meat samples were used to evaluate fat, protein, ash, and moisture content carried out according to the procedures (AOAC, 2000). Frozen samples were placed at 2°C for 24 hours before being processed in a meat grinder with a 3 mm (1/8”) cutting plate (Cabelas PRO 450, Sidney, NE 69160).
Determination of some biochemical parameters
Concurrently at slaughter, seven blood samples from each treatment were collected in non-heparinized test tubes. They were immediately centrifuged for separating blood serum. Sera were frozen at – 20ºC for later analysis. Blood glucose levels, protein (total), albumin, calcium, and uric acid measured with a spectrophotometer (SELECTA® UV-2005) using a commercial detection kit (Bio-diagnostic, Egypt) as directed by the manufacturer. Subtracting the albumin value from the total protein value of the same sample yielded serum globulin levels (Coles, 1986).The serum levels of IgA, IgG, and IgM were measured using Elabscience Co’s ELISA kits.
Determination of caecum microflora
Seven birds were chosen at random and euthanized by cutting the jugular vein. The carcasses were opened, and the whole gastrointestinal tract was aseptically removed. Before separation, the GI tract was separated into parts and ligated with light wire. The ceca were sealed and placed in sterile bags filled with ice-cold cryoprotective broth (50 mL), which was used to keep gut flora alive (Ballongue 1997) and stored at -80° until analyses. For all analytical techniques, deep-frozen ceca were thawed for 20 minutes and removed from storage bags. The contents of the cecal digest were then aseptically emptied into a new sterile bag and diluted 10-fold (10 % wt/vol) with sterile ice-cold anoxic PBS (0.1 M, PH 7.0) before being homogenised for
Table 2: Impacts of STE addition on the growth performance of broiler chicks.
| Items | STE (g/kg diet) | SEM | P-value | |||
| 0 | 1.5 | 3 | 6 | |||
| Body weight 19 d | 547.73 | 550.53 | 549.17 | 547.53 | 1.31 | 0.31 |
| Body weight 35 d |
1812.80c |
1937.60b |
1951.08b |
1995.70a |
7.28 | 0.01 |
| Body weight gain (19-35d) |
1265.07c |
1387.07bc |
1401.91ab |
1448.16a |
7.19 | 0.01 |
| Feed intake (19-35d) |
2412.35b |
2518.78b |
2525.26b |
2606.87a |
11.69 | 0.01 |
| Feed conversion ratio (19-35d) |
1.91a |
1.82b |
1.80b |
1.80b |
0.03 |
0.01 |
a,b Means in a row with no common superscripts differ (p≤0.05)
Table 3: Impacts of STE addition on carcass traits of broiler chicks.
| Items (% live body weight) | STE (g/kg diet) | SEM | P-value | |||
| 0 | 1.5 | 3 | 6 | |||
| Carcass | 71.53 | 71.77 | 71.36 | 72.12 | 0.10 | 0.40 |
| Breast | 32.08 | 32.21 | 32.22 | 33.08 | 0.74 | 0.14 |
| Thigh | 26.62 | 27.08 | 26.72 | 27.16 | 0.63 | 0.15 |
|
Fillet |
16.50 | 16.57 | 16.57 | 16.82 | 0.08 | 0.60 |
| Tender | 2.99 | 3.01 | 3.01 | 3.05 | 0.05 | 0.88 |
| Thigh meat | 14.13 | 14.36 | 14.77 | 14.81 | 0.07 | 0.21 |
| Wings | 6.83 | 6.80 | 6.91 | 6.88 | 0.03 | 0.40 |
| Abdominal fat |
1.07a |
0.99b |
0.99b |
0.97b |
0.007 |
0.01 |
a,b Means in a row with no common superscripts differ (p≤0.05)
Table 4: Impacts of STE addition meat quality of broiler chicks.
| Carcass parts | Items | STE (g/kg diet) | SEM | P-value | |||
| 0 | 1.5 | 3 | 6 | ||||
| Breast | Shear-value (kg/g sample) |
2.93c |
3.23b |
3.58a |
3.69a |
0.05 |
0.01 |
| Cooking loss (%) | 24.12 | 23.85 | 23.62 | 23.57 | 0.04 | 0.60 | |
|
Thigh |
Moisture (%) | 73.52 | 73.36 | 73.25 | 73.18 | 0.03 |
0.75 |
| Protein (%) | 85.60 | 86.00 | 86.19 | 86.25 | 0.05 |
0.51 |
|
| Fat (%) | 9.95 | 9.67 | 9.60 | 9.23 | 0.05 |
0.49 |
|
| Ash (%) | 4.61 | 4.57 | 4.56 | 4.57 | 0.01 |
0.54 |
|
a,b Means in a row with no common superscripts differ (p≤0.05)
Table 5: Impacts of STE addition on some biochemical parameters of broiler chicks.
|
Items |
STE (g/kg diet) | SEM | P-value | |||
| 0 | 1.5 | 3 | 6 | |||
|
Glucose (mg L-1) |
42.02a |
39.39b |
37.90c |
36.54d |
0.33 | 0.01 |
|
Total protein (mg L-1) |
39.05c |
41.71b |
42.11b |
43.16a |
0.51 | 0.01 |
|
Albumin (mg L-1) |
20.83 | 20.20 | 20.67 | 21.04 | 0.08 | 0.70 |
|
Globulin (mg L-1) |
18.51c |
19.97b |
20.31b |
21.13a |
0.11 | 0.01 |
|
Calcium (mg L-1) |
109.40 | 113.55 | 113.99 | 115.96 | 1.10 | 0.21 |
|
Uric acid (mg L-1) |
73.20 | 76.11 | 71.53 | 75.79 | 3.02 | 0.67 |
|
IgA (mg ml-1) |
1.20c |
1.26b |
1.27b |
1.33a |
0.04 | 0.02 |
|
IgG (mg ml-1) |
3.40d |
3.56c |
3.69b |
3.83a |
0.02 | 0.01 |
|
IgM (mg ml-1) |
1.49 | 1.49 | 1.50 | 1.50 | 0.02 |
0.25 |
a,b Means in a row with no common superscripts differ (p≤0.05)
Table 6: Impacts of STE addition on ceacum microflora count of broiler chicks.
|
Items
(log 10 cfu g-1) |
STE (g/kg diet) | SEM | P-value | |||
| 0 | 1.5 | 3 |
6 |
|||
| Lactobacillus spp. |
6.53b |
6.57b |
6.76b |
7.00a |
0.03 |
0.05 |
| Escherichia coli |
5.77a |
5.49b |
5.42b |
5.25c |
0.04 |
0.01 |
| Salmonella spp. | 2.25 | 2.24 | 2.16 | 2.19 | 0.01 |
0.11 |
a,b Means in a row with no common superscripts differ (p≤0.05)
3 minutes. The digesta slurries were then treated in the following manner. Each cecal digest homogenate was serially diluted in PBS (1 mL) from 10-1 to 10-7.The bacterial target groups were then counted by plating dilutions on duplicate selective agar media M.R.S, MacConkey agar, and salmonella shigella agar were used to count lactobacillus spp., E. coli, and salmonella, respectively (Tuohy et al., 2002). Colonies were counted after plates were incubated at 39° for 24 to 72 hours.The colony forming units per grams of cecal digest were calculated using a base-10 logarithm.
Statistical Analysis
A completely statistical randomized design was used in this study. Using the Statistical Package for the Social Sciences SPSS (2008), data were statistically analysed using one-way analysis of variance. Duncan’s multiple range test (Duncan, 1955) was used to determine whether there were significant differences between the means of the variables. The following mathematical model was applied:
Yij = 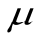 + Ti + eij
+ Ti + eij
Where: Yij = Observed value of the dependent variable.
µ = Overall mean.
T = effect of treatments (STE addition).
eij = experimental random error.
Results
Growth performance
Table 2 shows the effect of dietary STE levels on the GP of broiler chicks during the finisher period (19-35d). Results showed that the S6 group recorded the highest BW (p< 0.01) compared to control group; also, S1.5 and S3 showed higher BW than the control. Furthermore, STE supplementation enhanced BWG and FI at (19-35d) considerably (p< 0.01) when compared to the control group. In terms of feed conversion, the results demonstrated that broilers given diets supplemented with STE at various doses performed better than the control group.
Carcass traits and meat quality
Tables 3 and 4 show the results of carcass characteristics and meat quality. No significant difference was observed in all carcass characteristics among treatments except abdominal fat which decreased significantly (p< 0.01) when diets supplemented with STE at different levels than the control group.
Shear-values of breast meat were affected positively (p<0.01) by STE addition, birds were fed 6 and 3g STE/kg feed showed the highest shear-value compared to the control group.
In terms of cooking loss of breast meat and thigh meat parameters (moisture, protein, and ash content), no differences (p > 0.05) were found among treatments.
Blood biochemical parameters
Data in Table 5 represented some biochemical blood constituents; serum glucose levels were decreased significantly (p<0.01) by STE addition compared with the control group. Concerning plasma total protein and globulin, it could be speculated that the STE supplemental significantly (p<0.01) increased both of them compared to control group. While albumin, calcium, and uric acid concentrations showed no significant difference among the groups supplied with STE and the control group.
The data shows that supplementation diets with STE caused a significant (p< 0.01) increase in plasma IgA and IgG values compared with control group.
Caecum microflora
Table 6 shows that dietary STE supplementation reduced E. coli concentrations (P = 0.01), increased Lactobacillus concentrations (p = 0.05), and had no effect on Salmonella concentrations (P = 0.11).
Discussion
Results of GP coincided with the results reported by Atteh et al. (2008), who concluded that STE addition enhanced the average BWG of broiler chicks. Also, Jiang et al. (2020) mentioned that dietary addition with STE increased the GP of broiler chicks. Inversely, Wu et al. (2019) found that STE addition to 3200 mg/ kg diet did not affect GP of broilers through a 42d study. Significant elevation of the FI reported in the present study could be attributed to chickens’ insensitivity to sweet substances. Similar result was found by Shi and Zhang (2006), and STE supplementation enhanced the FI, which, in turn, increased the GP of broilers chicks at finisher period. The distribution of microflora in the cecum of broiler chickens has been suggested to be affected by STE supplementation (Wu et al., 2019). The microbiota intestines play a remarkable role in making neuropeptides and short-chain fatty acids, which might affect the FI of birds (Cryan et al., 2019: Metzler-Zebeli et al., 2019).
Results also showed that abdominal fat was decreased when diets were supplemented with STE; these alterations in fat deposition most likely resulted from alterations in lipid metabolism (Cherian et al.,, 2002). The less accumulation of abdominal fat may be due to gut microflora which outputs short-chain fatty acids which reduce the accumulation of fats on the carcass (Metzler-Zebeliet al., 2019).
The absolute values of the meat quality indices of the breast muscles in this investigation were within the data range by (Nissen and Young 2006; Werner et al., 2009). Higher shear force values were found, indicating that they will be well received by customers (Corzo et al., 2009).
There were no changes in the cooking loss of breast meat, which is similar to the findings of (Meek et al., 2000) but slightly greater than other studies (Corzo et al., 2009; Schilling et al., 2010), which found cooking losses of around 20%. During cooking, weight loss may vary due to differences in lipid content and pH (Souza et al.,, 2011). It means that STE supplementation has no detrimental impact on the quality of the meat.
Results of blood glucose concentration are in agreement with Atteh et al. (2008) who stated that STE supplementation at 2% resulted in a significant decrease in blood glucose in broilers. Also, Wu et al. (2019) revealed that STE addition at different doses decreased glucose levels of broilers. It’s possible that STE ability to lower blood glucose levels without causing hypoglycemia is the reason for the considerable decline in blood glucose levels following therapy- 1) increase the action of insulin on cells, 2) establish glucagon secretion and blood sugar levels, 3) increase insulin production, and 4) enhance glucose tolerance for carbohydrate absorption in animals and lower post-prandial blood sugar levels (Chen et al., 2005; 2006).
The increase in total protein and globulin with STE addition may be due to increased protein synthesis. The obtained results were supported by Jiang et al. (2020), who found that the addition of STE at 250 mg/kg broilers diet significantly increased total protein concentration. These findings, on the other hand, are in contrast to those of Wu et al. (2019), who found that STE supplementation had no effect on total serum protein. Results showed no significant effect on serum uric acid and calcium, as obtained by Jiang et al. (2020). They indicated that no action of sweeteners addition on serum uric acid in broiler chickens at 21d. Inversely, Wu et al. (2019) found that the addition of STE at 1600 and 3200 mg/kg diet significantly increased serum calcium in broiler chickens.
Results of serum immunoglobulin levels agree with Wu et al. (2019) who reported that STE supplementation resulted in a significant increase in serum IgG and IgA levels in broilers. The improvement in immunological status may be due to STE has constituents with several biological properties such as antimicrobial, anti-inflammatory, immunomodulatory, and antioxidant effects (Jaroslav et al., 2006; Satishkumar et al., 2008). Furthermore, dietary supplementation with STE increased blood concentration levels of total proteins and globulin, suggesting that STE could improve broiler chicken immunology and protein synthesis. This conclusion was in agreement with the increase in growth performance. Animals could not digest STE (Hutapea et al., 1997),but the gut bacteria content of broilers produces enzymes that can break it down (Geuns et al., 2003). Bacteria can convert STE to steviol, according to Hutapea et al. (1997). The current research suggests that STE can help Lactobacillus grow in the caecum tract. Lactobacillus may have a variety of health benefits through maintaining gut microbial equilibrium (Yuan et al., 2018)
In summary, STE supplementation improves GP, abdominal fat content, shear-value of breast meat, some blood parameters, and cecal digesta content of broilers positively and can be used as a feed additive in finisher broiler diets.
Conflict of interest
No conflict of interest.
authors contribution
AMK & WMD: Experiment idea and design. ASE, MIH & WAK: Executing the experiment and lab analysis. AMK: Statistical analysis. AMK & ASE: Write the manuscript. WMD: revised the manuscript.
References






