Advances in Animal and Veterinary Sciences
Research Article
Fish Diversity of Coastal Andhra Pradesh, Southeast Coast of India
Darwin Chatla, P. Padmavathi*
Department of Zoology and Aquaculture, Acharya Nagarjuna University, Nagarjuna Nagar – 522 510, Andhra Pradesh, India.
Abstract | Diversity of marine fishes was studied along the coast of Bay of Bengal from four selected landing stations of Andhra Pradesh, Southeast coast of India during the period from December 2016 to November 2018. A total of 171 species belonging to 14 orders, 63 families and 128 genera have been recorded during the study period. It is evident that marine fish production is well below the production targets. The less availability of some species indicates a remarkable decline in the diversity of fishes. The anthropogenic disturbances and climatic changes are reported to be the factors affecting the fish population and diversity. Therefore, we are in the stage of need of the hour to conserve marine biodiversity in coastal Andhra Pradesh. The current study also recorded the IUCN status of 171 fish species in various categories of conservation status.
Keywords | Bio-diversity, Conservation, IUCN status, Marine fisheries, Andhra Pradesh
Received | March 10, 2021; Accepted | April 26, 2021; Published | July 28, 2021
*Correspondence | P. Padmavathi, Department of Zoology and Aquaculture, Acharya Nagarjuna University, Nagarjuna Nagar – 522 510, Andhra Pradesh, India; Email: [email protected]
Citation | Chatla D, Padmavathi P (2021). Fish diversity of coastal Andhra Pradesh, southeast coast of India. Adv. Anim. Vet. Sci. 9(9): 1424-1436.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1424.1436
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Chatla and Padmavathi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
India is one of the largest mega-diversity countries and known for its biodiversity richness abundance reflected in the diversity of fresh and marine water fishes. It is well-known that the marine faunal diversity depends mostly on fish diversity (Kar et al., 2017). It was estimated that India housed 3231 valid species, of which 2443 are marine (75.6%) (Gopi and Mishra, 2015). Globally, India occupies the second-largest fish producer with 3.56 million tonnes for the year 2019 (FRAD, 2019) and exports worth US $7.08 billion (DADF, 2019). The fisheries play a remarkable role in the agro-economy of India with regard to protein supply and employment (Chatla et al., 2020).
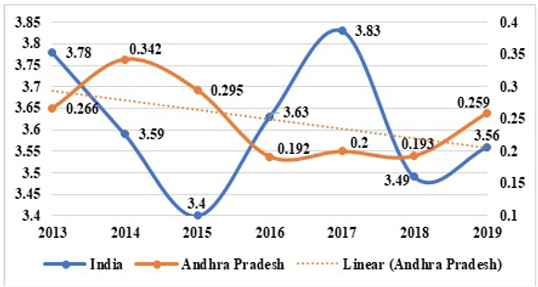
The state of Andhra Pradesh endowed with a vast coastline of 974 km spread across nine coastal districts is situated between 150 54’ 46.44” N latitude and 790 44’ 23.95” E longitude. It is scattered with 353 fish landing stations including 4 major harbors viz., Visakhapatnam, Kakinada, Machilipatanam, and Nizampatnam (CMFRI, 2012). Andhra Pradesh has known for its healthy fishing grounds and diverse resources comprising different gears and crafts with a total number of 27211 fishing vessels, including 1675 mechanized, 11807 motorized and 13729 traditional fishing crafts, and gears such as seines, cast nets, drag nets, gill nets, trawl nets, and hook and lines (Rao et al., 2008; WAPCOS, 2017). The marine fish landings accounted for about 0.259 million tonnes (mt) in 2019 (FRAD, 2020). However, fish landings have fluctuated over the years from 2013 to 2019 (Figure 1).
Every region of the sea is a home for wide variety of life and repository of biodiversity. Among marine biodiversity, fish diversity is comparatively higher than other faunal diversity with ample existing data and higher probability for the discovery of new species (Pyle et al., 2019). The dispersion patterns of several species and extinction of indigenous fishes have been directly linked to human interference (Nelson et al., 2016). Of late, over exploitation of fish species has become a matter of great concern (Ranjan, 2018). The distribution of fishery resources in the coastal waters varies with distance from the shore (Vardharajan and Soundarapandian, 2015). The catchment rates vary with the landing stations and the species (Kar et al., 2017), and hence the catchment information is essential for the development of effective management and to develop the measures to be taken to conserve the fishes towards sustainable utilization (Darwin and Padmavathi, 2020). In view of the given significance of diversity, the fishery composition of landings and seasonal abundance of different resources along the Visakhapatnam coast were studied by Sudarsan (1981), Krishnan and Mishra (1993), Sujatha (1995), Barman et al. (2004) and Sreedhar et al. (2009). However, there is no documented evidence on the diversity of fishes in other fish landing stations of coastal Andhra Pradesh. Therefore, the current study has been undertaken to know the marine fish diversity of unexplored four fish landing stations of coastal Andhra Pradesh.
MATERIALS AND METHODS
Study Area
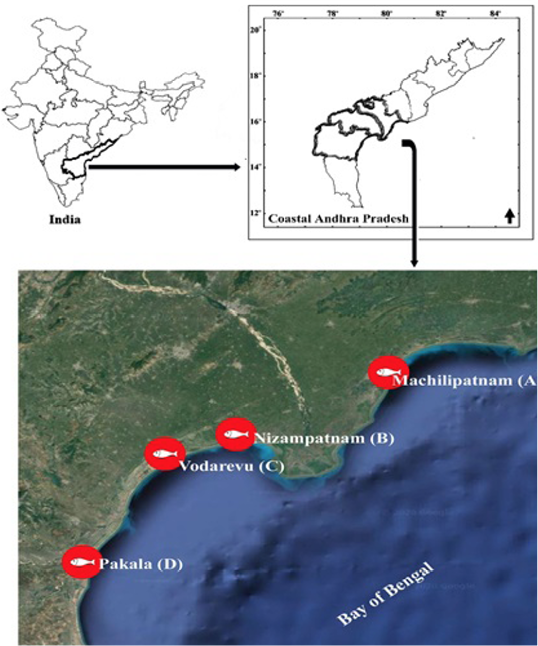
The present study is based on the samples collected in four designated landing stations of coastal Andhra Pradesh viz., Machilipatnam (A), Nizampatnam (B), Vodarevu (C), and Pakala (D) (Figure 2) located in Krishna, Guntur, and Prakasam districts along 224 km coastline (Table 1 and 2).
Sample Collection
To study the marine fish diversity, fish samples were collected from the catches of fish landing centres where fishes were caught by using different gears such as cast nets, trammel net, hook and line, shore seine, boat seine and purse seine. Sampling was done at regular fortnight or monthly intervals in all four landing stations from December 2016 to November 2018 except fish ban period of the months of April and May. The fish samples from various catches were collected as soon as the catches were offloaded. Some of the samples were preserved in 5% formalin and transported to the laboratory for taxonomic identification. All the collections after identification were documented at Museum, Department of Zoology and Aquaculture, Acharya Nagarjuna University, Andhra Pradesh, India.
Table 1: Geographical location of the four sampling sites of coastal Andhra Pradesh.
| Fish landing stations | Geographical location of fish landing stations | |
| Latitude (North) | Longitude (East) | |
| Machilipatnam (A) |
160 14’ 49” |
810 18’ 63” |
| Nizampatnam (B) |
150 52’ 58” |
800 38’ 18” |
| Vodarevu (C) |
150 79’ 34” |
800 41’ 10” |
| Pakala (D) |
150 27’ 31” |
800 08’ 53” |
Fish Diversity and Abundance
Fishes were identified up to the species level by following the standard books (Talwar and Kacker, 1984; Barman et al., 2004) and web-based keys, FishBase (www.fishbase.in) (Froese and Pauly, 2020), and Eschmeyer’s Catalog of Fishes (www.calacademy.org) (Fricke et al., 2020). The classification adopted was mainly followed by Nelson (2006). The current valid names, common names and concise data on the conservation status of fish species and importance to fisheries was gathered based on the FishBase (Froese and Pauly, 2020), International Union for Conservation of Nature (www.iucn.org) (IUCN, 2019) and World Register of Marine Species (www.marinespecies.org) (WoRMS, 2020). The percentage composition of orders, families, genera and species of collected fishes was recorded.
Table 2: fish species, Common names, Threat to humans, IUCN status and occurrence stations in coastal Andhra Pradesh.
|
Class: Chondrichthyes/Elasmobranchii |
|||||||||
|
S. No. |
Order |
Family |
Species Name |
Common Name |
Station |
IUCN Status |
Threat to Humans |
Human Use |
|
|
1 |
Carcharhiniformes |
Carcharhinidae |
Carcharhinus dussumieri (Müller & Henle, 1839) |
White cheek shark |
A, B |
EN |
H |
C |
|
|
2 |
|
|
C. limbatus (Müller & Henle, 1839) |
Blacktip shark |
A, B, C |
VU |
Tr |
C, Gf |
|
|
3 |
|
|
C. sorrah (Müller & Henle, 1839) |
Spot-tail shark |
A, B, C |
NT |
H |
C |
|
|
4 |
|
|
Lamiopsis temminckii (Müller & Henle, 1839) |
Broadfin Shark |
A, B |
EN |
H |
C |
|
|
5 |
|
|
Rhizoprionodon acutus (Rüppell, 1837) |
Milk shark |
A, B, C |
LC |
H |
C |
|
|
6 |
|
|
Scoliodon laticaudus Müller & Henle, 1838 |
Spadenose shark |
A, B |
NT |
H |
C |
|
|
7 |
|
Sphyrnidae |
Sphyrna zygaena (Linnaeus, 1758) |
Hammer head shark |
A, B, C, D |
VU |
Tr |
C, Gf |
|
|
8 |
Myliobatiformes |
Aetobatidae |
Aetobatus flagellum (Bloch & Schneider, 1801) |
Longheaded eagle ray |
A, B |
EN |
H |
C |
|
|
9 |
|
Dasyatidae |
Himantura fava (Annandale, 1909) |
Honeycomb whipray |
A, B |
NE |
H |
Ss |
|
|
10 |
|
|
H. uarnak (Gmelin, 1789) |
Honeycomb stingray |
A, B |
VU |
Tr |
C, Gf |
|
|
11 |
|
|
Maculabatis gerrardi (Gray, 1851) |
Sharp nose stingray |
A, B, C, D |
VU |
H |
C, Gf |
|
|
12 |
|
|
Telatrygon zugei (Müller & Henle, 1841) |
Pale-edged stingray |
A, B |
NT |
H |
C |
|
|
13 |
Orectolobiformes |
Hemiscylliidae |
Chiloscyllium griseum Müller & Henle, 1838 |
Grey bambooshark |
A, B, C, D |
NT |
H |
C |
|
|
14 |
Rhinopristiformes |
Rhinidae |
Rhynchobatus djiddensis (Forsskål, 1775) |
Giant guitarfish |
A, B |
VU |
H |
C, Gf |
|
|
15 |
|
Rhinobatidae |
Rhinobatos annandalei Norman, 1926 |
Annandale's guitarfish |
B |
DD |
H |
F |
|
|
16 |
Torpediniformes |
Narcinidae |
Narcine brunnea Annandale, 1909 |
Brown electric ray |
A, B |
NE |
H |
Ss |
|
|
17 |
|
Narkidae |
Narke dipterygia (Bloch & Schneider, 1801) |
Numbray |
A, B |
DD |
H |
C |
|
|
Class: Osteichthyes/Actinopterygii |
|||||||||
|
18 |
Anguilliformes |
Muraenidae |
Strophidon sathete (Hamilton, 1822) |
Slender giant moray |
B |
NE |
Tr |
C, Gf |
|
|
19 |
|
Muraenesocidae |
Muraenesox bagio (Hamilton, 1822) |
Common pike conger |
A, B |
NE |
H |
C, Gf |
|
|
20 |
|
|
Congresox talabonoides (Bleeker, 1853) |
Indian pike conger |
A, B, C, D |
NE |
H |
C |
|
|
21 |
|
Ophichthidae |
Lamnostoma orientalis (McClelland, 1844) |
Oriental worm-eel |
B, C |
LC |
H |
C |
|
|
22 |
Aulopiformes |
Synodontidae |
Harpadon nehereus (Hamilton, 1822) |
Bombay duck |
A, B, C, D |
NE |
H |
C |
|
|
23 |
|
|
Saurida tumbil (Bloch, 1795) |
Greater lizardfish |
A, B, C, D |
LC |
H |
C |
|
|
24 |
Beloniformes |
Belonidae |
Xenentodon cancila (Hamilton, 1822) |
Needle fish |
C, D |
LC |
Tr |
C, Aq |
|
|
25 |
|
|
Strongylura strongylura (vanHasselt, 1823) |
Spottail needlefish |
A, B, C, D |
NE |
H |
C, Gf |
|
|
26 |
|
Hemirhamphidae |
Hemiramphus marginatus (Forsskål, 1775) |
Yellowtip halfbeak |
B, C, D |
NE |
H |
Ss |
|
|
27 |
|
Zenarchopteridae |
Rhynchorhamphus georgii (Valenciennes, 1847) |
Long billed half beak |
C, D |
NE |
H |
C |
|
|
28 |
Clupeiformes |
Chirocentridae |
Chirocentrus dorab (Forsskål, 1775) |
Dorab wolf-herring |
A, B, C, D |
LC |
H |
C, Gf |
|
|
29 |
|
|
C. nudus Swainson, 1839 |
Whitefin wolf-herring |
A, B |
LC |
H |
C |
|
|
30 |
|
Clupeidae |
Hilsa kelee (Cuvier, 1829) |
Kelee shad |
A, B, C, D |
LC |
H |
C |
|
|
31 |
|
|
Sardinella brachysoma Bleeker, 1852 |
Deep body sardinella |
B, C, D |
NE |
H |
C |
|
|
32 |
|
|
S. fimbriata (Valenciennes, 1847) |
Fringescale sardinella |
A, B, C, D |
LC |
H |
C |
|
|
33 |
|
|
S. gibbosa (Bleeker, 1849) |
Goldstripe sardinella |
B, C |
LC |
H |
C |
|
|
34 |
|
|
S. longiceps Valenciennes, 1847 |
Indian oil sardine |
A, B, C, D |
LC |
H |
C |
|
|
35 |
|
|
Tenualosa ilisha (Hamilton, 1822) |
Hilsa shad |
A, B, C, D |
NE |
H |
C, Ac |
|
|
36 |
|
Engraulidae |
Coilia dussumieri Valenciennes, 1848 |
Goldspotted grenadier anchovy |
A, B, C, D |
LC |
H |
C |
|
|
37 |
|
|
Encrasicholina devisi (Whitley, 1940) |
Devis' anchovy |
A, B, C, D |
NE |
H |
C |
|
|
38 |
|
|
Stolephorus commersonnii Lacepède, 1803 |
Commerson's anchovy |
A, B, C, D |
LC |
H |
C |
|
|
39 |
|
|
S. indicus (vanHasselt, 1823) |
Indian anchovy |
A, B, C, D |
LC |
H |
C |
|
|
40 |
|
|
Thryssa dussumieri (Valenciennes, 1848) |
Dussumier's thryssa |
A, B, C, D |
LC |
H |
C |
|
|
41 |
|
|
T. hamiltonii Gray, 1835 |
Hamilton's thryssa |
C, D |
LC |
H |
C |
|
|
42 |
|
|
T. malabarica (Bloch, 1795) |
Malabar thryssa |
A, B, C, D |
DD |
H |
C |
|
|
43 |
|
|
T. mystax (Bloch & Schneider, 1801) |
Moustached thryssa |
A, B, C, D |
LC |
H |
C |
|
|
44 |
|
|
T. setirostris (Broussonet, 1782) |
Longjaw thryssa |
A, B, C, D |
LC |
H |
C |
|
|
45 |
|
Pristigasteridae |
Amblygaster leiogaster (Valenciennes, 1847) |
Smoothbelly sardinella |
B, C, D |
LC |
H |
F |
|
|
46 |
|
|
Anodontostoma chacunda (Hamilton, 1822) |
Chacunda gizzard shad |
A, B, C, D |
LC |
H |
C |
|
|
47 |
|
|
Ilisha melastoma (Bloch & Schneider, 1801) |
Indian ilisha |
A, B |
LC |
H |
C |
|
|
48 |
|
|
Nematalosa nasus (Bloch, 1795) |
Bloch's gizzard shad |
A, B, C, D |
LC |
H |
C |
|
|
49 |
|
|
Opisthopterus tardoore (Cuvier, 1829) |
Tardoore |
B, D |
LC |
H |
C |
|
|
50 |
Elopiformes |
Elopidae |
Elops machnata (Forsskål, 1775) |
Tenpounder |
A, B, D |
LC |
H |
C, Gf |
|
|
51 |
Mugiliformes |
Mugilidae |
Chelon parsia (Hamilton, 1822) |
Goldspot mullet |
A, B, C, D |
NE |
H |
C |
|
|
52 |
|
|
C. planiceps (Valenciennes, 1836) |
Tade gray mullet |
B, C |
NE |
H |
C |
|
|
53 |
|
|
Mugil cephalus Linnaeus, 1758 |
Flathead grey mullet |
A, B, C, D |
LC |
H |
C, Ac |
|
|
54 |
|
|
Planiliza macrolepis (Smith, 1846) |
Largescale mullet |
A, B, C, D |
LC |
H |
C, Ac |
|
|
55 |
Perciformes |
Acanthuridae |
Acanthurus mata (Cuvier, 1829) |
Elongate surgeonfish |
A, B |
LC |
Vn |
C, Aq |
|
|
56 |
|
Ambassidae |
Ambassis nalua (Hamilton, 1822) |
Scalloped perchlet |
A, B |
LC |
H |
Ukn |
|
|
57 |
|
|
Chanda nama Hamilton, 1822 |
Elongate glass-perchlet |
C, D |
LC |
H |
C, Aq |
|
|
58 |
|
Apogonidae |
Fibramia lateralis (Valenciennes, 1832) |
Humpback cardinal |
C, D |
LC |
H |
Ss |
|
|
59 |
|
Carangidae |
Atropus atropos (Bloch & Schneider, 1801) |
Cleft belly trevally |
A, B, C, D |
LC |
H |
C |
|
|
60 |
|
|
Alectis indica (Rüppell, 1830) |
Indian threadfish |
A, B |
LC |
H |
C, Gf |
|
|
61 |
|
|
Carangoides malabaricus (Bloch & Schneider, 1801) |
Malabar trevally |
A, B |
LC |
H |
C, Gf |
|
|
62 |
|
|
Caranx ignobilis (Forsskål, 1775) |
Giant trevally |
A, B |
LC |
Pn |
C, Gf |
|
|
63 |
|
|
C. sexfasciatus Quoy & Gaimard, 1825 |
Bigeye trevally |
A |
LC |
H |
C |
|
|
64 |
|
|
Decapterus russelli (Rüppell, 1830) |
Indian scad |
A, B, C, D |
LC |
H |
C |
|
|
65 |
|
|
Parastromateus niger (Bloch, 1795) |
Black pomfret |
A, B, C, D |
LC |
H |
C |
|
|
66 |
|
|
Selar crumenophthalmus (Bloch, 1793) |
Bigeye scad |
A, B |
LC |
Pn |
C, Gf |
|
|
67 |
|
|
Trachinotus blochii (Lacepède, 1801) |
Indian pompano |
A |
LC |
Pn |
C, Gf |
|
|
68 |
|
Cynoglossidae |
Cynoglossus bilineatus (Lacepède, 1802) |
Fourlined tonguesole |
A, B |
NE |
H |
C |
|
|
69 |
|
|
C. cynoglossus (Hamilton, 1822) |
Bengal tongue sole |
A, B, C, D |
NE |
H |
C |
|
|
70 |
|
|
C. macrolepidotus (Bleeker, 1851) |
Large scale tongue sole |
A, B |
NE |
H |
Ss |
|
|
71 |
|
|
C. semifasciatus Day, 1877 |
Bengal tonguesole |
A, B |
NE |
H |
Ss |
|
|
72 |
|
|
Paraplagusia bilineata (Bloch, 1787) |
Doublelined tonguesole |
A, B, C, D |
NE |
H |
C |
|
|
73 |
|
Derepanidae |
Drepane punctata (Linnaeus, 1758) |
Spotted sicklefish |
A, B, C, D |
NE |
H |
C, Aq |
|
|
74 |
|
Ephippidae |
Ephippus orbis (Bloch, 1787) |
Orbfish |
B |
NE |
H |
C |
|
|
75 |
|
Gerridae |
Gerres filamentosus Cuvier, 1829 |
Whipfin silver-biddy |
B, C, D |
LC |
H |
C |
|
|
76 |
|
|
G. setifer (Hamilton, 1822) |
Small Bengal silverbiddy |
A, B |
NE |
H |
C |
|
|
77 |
|
Gobiidae |
Boleophthalmus dussumieri Valenciennes, 1837 |
Mudskipper |
C, D |
LC |
H |
Ukn |
|
|
78 |
|
|
P. novemradiatus (Hamilton, 1822) |
Pearse's mudskipper |
B, C, D |
DD |
H |
Ukn |
|
|
79 |
|
Haemulidae |
Pentaprion longimanus (Cantor, 1849) |
Longfin mojarra |
A, B |
LC |
H |
C |
|
|
80 |
|
|
Pomadasys maculatus (Bloch, 1793) |
Saddle grunt |
A |
LC |
H |
C |
|
|
81 |
|
Istiophoridae |
Istiophorus platypterus (Shaw, 1792) |
Indo-Pacific sailfish |
A, B, C, D |
LC |
H |
C, Gf |
|
|
82 |
|
|
Istiompax indica (Cuvier, 1832) |
Black marlin |
A, B |
DD |
H |
C, Gf |
|
|
83 |
|
Lactariidae |
Lactarius lactarius (Bloch & Schneider, 1801) |
False trevally |
A, B |
NE |
H |
C |
|
|
84 |
|
Latidae |
Lates calcalifer (Bloch, 1790) |
Barramundi |
A, B, C, D |
NE |
H |
C, Ac |
|
|
85 |
|
Leiognathidae |
Eubleekeria splendens (Cuvier, 1829) |
Splendid ponyfish |
A, B |
LC |
H |
C |
|
|
86 |
|
|
Leiognathus equulus (Forsskål, 1775) |
Common ponyfish |
A, B, C, D |
LC |
H |
C |
|
|
87 |
|
|
Karalla dussumieri (Valenciennes, 1835) |
Dussumier’s ponyfish |
A, B |
NE |
H |
C |
|
|
88 |
|
|
Nuchequula gerreoides (Bleeker, 1851) |
Decorated ponyfish |
B |
NE |
H |
Ss |
|
|
89 |
|
|
Photopectoralis bindus (Valenciennes, 1835) |
Orangefin ponyfish |
A, B |
NE |
H |
C |
|
|
90 |
|
|
Secutor insidiator (Bloch, 1787) |
Pugnose ponyfish |
A, B |
NE |
H |
C |
|
|
91 |
|
|
S. ruconius (Hamilton, 1822) |
Deep pugnose ponyfish |
A, B |
NE |
H |
C |
|
|
92 |
|
Lobotidae |
Lobotes surinamensis (Bloch, 1790) |
Tripletail |
A, B |
LC |
H |
C, Gf |
|
|
93 |
|
Lutjanidae |
Lutjanus argentimaculatus (Forsskål, 1775) |
Mangrove red snapper |
A |
LC |
Pn |
C, Gf |
|
|
94 |
|
|
L. indicus Allen, White & Erdmann, 2013 |
Snapper fish |
A, B |
LC |
H |
Ss |
|
|
95 |
|
|
L. johnii (Bloch, 1792) |
John’s snapper |
A, B, C, D |
NE |
H |
C, Gf |
|
|
96 |
|
|
L. russellii (Bleeker, 1849) |
Russell’s snapper |
A, B |
NE |
H |
C |
|
|
97 |
|
Menidae |
Mene maculate (Bloch & Schneider, 1801) |
Moonfish |
A, B |
NE |
H |
C |
|
|
98 |
|
Mullidae |
Parupeneus indicus (Shaw, 1803) |
Indian goatfish |
A, B |
LC |
H |
C, Gf |
|
|
99 |
|
|
Upeneus vittatus (Forsskål, 1775) |
Yellow striped goatfish |
A, B, C, D |
LC |
H |
C, Aq |
|
|
100 |
|
Nemipteridae |
Nemipterus japonicus (Bloch, 1791) |
Japanese threadfin bream |
A, B, C, D |
LC |
H |
C |
|
|
101 |
|
|
N. bipunctatus (Valenciennes, 1830) |
Delagoa threadfin bream |
A, B |
LC |
H |
C |
|
|
102 |
|
Platycephalidae |
Platycephalus indicus (Linnaeus, 1758) |
Bartail flathead |
A, B |
DD |
H |
C, Gf |
|
|
103 |
|
Polynemidae |
Polynemus paradiseus Linnaeus, 1758 |
Paradise threadfin |
A, B |
LC |
H |
C |
|
|
104 |
|
|
Polydactylus plebeius (Broussonet, 1782) |
Striped threadfin |
A, B |
NE |
H |
C, Gf |
|
|
105 |
|
|
Eleutheronema tetradactylum (Shaw, 1804) |
Fourfinger threadfin |
A, B, C, D |
NE |
H |
C, Gf |
|
|
106 |
|
|
Leptomelanosoma indicum (Shaw, 1804) |
Indian threadfin |
A, B |
NE |
H |
C, Gf |
|
|
107 |
|
Priacanthidae |
Priacanthus hamrur (Forsskål, 1775) |
Moontail bullseye |
A, B, C, D |
LC |
H |
C, Aq |
|
|
108 |
|
Psettodidae |
Pseudorhombus arsius (Hamilton, 1822) |
Largetooth flounder |
A, B |
NE |
H |
C, Gf |
|
|
109 |
|
|
Psettodes erumei (Bloch & Schneider, 1801) |
Indian halibut |
A, B, C, D |
NE |
H |
C |
|
|
110 |
|
Rachycentridae |
Rachycentron canadum (Linnaeus, 1766) |
Cobia |
A, B, C, D |
LC |
H |
C, Ac |
|
|
111 |
|
Scatophagidae |
Scatophagus argus (Linnaeus, 1766) |
Spotted scat |
A, B, C, D |
LC |
Pn |
C, Aq |
|
|
112 |
|
Sciaenidae |
Atrobucca nibe (Jordan & Thompson, 1911) |
Blackmouth croaker |
A, B |
NE |
H |
C |
|
|
113 |
|
|
Bahaba chaptis (Hamilton, 1822) |
Chaptis bahaba |
A, B |
DD |
H |
C |
|
|
114 |
|
|
Chrysochir aureus (Richardson, 1846) |
Reeve's croaker |
A, B, C, D |
NE |
H |
C |
|
|
115 |
|
|
Daysciaena albida (Cuvier, 1830) |
Bengal corvine |
A, B, C, D |
NE |
H |
C |
|
|
116 |
|
|
Dendrophysa russelii (Cuvier, 1829) |
Goatee croaker |
A, B, C, D |
NE |
H |
C |
|
|
117 |
|
|
Johnius belangerii (Cuvier, 1830) |
Belanger's croaker |
A, B, C, D |
NE |
H |
C |
|
|
118 |
|
|
Johnius carutta Bloch, 1793 |
Karut croaker |
A, B |
NE |
H |
C |
|
|
119 |
|
|
J. dussumieri (Cuvier, 1830) |
Sin croaker |
A, B |
NE |
H |
C |
|
|
120 |
|
|
Macrospinosa cuja (Hamilton, 1822) |
Cuja bola |
B, C |
NE |
H |
C |
|
|
121 |
|
|
Nibea coibor (Hamilton, 1822) |
Ganges jaw fish |
A, B, C, D |
NE |
H |
Ss |
|
|
122 |
|
|
N. maculate (Bloch & Schneider, 1801) |
Blotched croaker |
A, B, C, D |
NE |
H |
C |
|
|
123 |
|
|
Otolithes ruber (Bloch & Schneider, 1801) |
Tigertooth croaker |
A, B |
NE |
H |
C, Gf |
|
|
124 |
|
|
Protonibea diacanthus (Lacepède, 1802) |
Blackspotted croaker |
A, B |
NE |
H |
C |
|
|
125 |
|
Scombridae |
Auxis thazard (Lacepède, 1800) |
Frigate tuna |
A, B |
LC |
H |
C, Gf |
|
|
126 |
|
|
Euthynnus affinis (Cantor, 1849) |
Kawakawa |
A, B, C, D |
LC |
Pn |
C, Gf |
|
|
127 |
|
|
Katsuwonus pelamis (Linnaeus, 1758) |
Skipjack tuna |
A, B, C, D |
LC |
Pn |
C, Gf |
|
|
128 |
|
|
Rastrelliger kanagurta (Cuvier, 1816) |
Indian mackerel |
A, B, C, D |
DD |
H |
C, Gf |
|
|
129 |
|
|
Scomberomorus commerson (Lacepède, 1800) |
Barred Spanish mackerel |
A, B, C, D |
NT |
Pn |
C, Gf |
|
|
130 |
|
|
S. guttatus (Bloch & Schneider, 1801) |
IndoPacific king mackerel |
A, B, C, D |
DD |
H |
C, Gf |
|
|
131 |
|
|
S. lineolatus (Cuvier, 1829) |
Streaked seerfish |
A, B, C, D |
LC |
H |
C, Gf |
|
|
132 |
|
|
Thunnus albacares (Bonnaterre, 1788) |
Yellowfin tuna |
A, B, C, D |
NT |
H |
C, Gf |
|
|
133 |
|
|
T. obesus (Lowe, 1839) |
Bigeye tuna |
A, B |
VU |
H |
C, Gf |
|
|
134 |
|
|
T. tonggol (Bleeker, 1851) |
Longtail tuna |
A, B |
DD |
H |
C, Gf |
|
|
135 |
|
Serranidae |
Cephalopholis aurantia (Valenciennes, 1828) |
Golden hind |
A |
LC |
H |
C |
|
|
136 |
|
|
Cephalopholis formosa (Shaw, 1812) |
Blue lined hind |
A, B, C, D |
LC |
H |
Sf |
|
|
137 |
|
|
Cromileptes altivelis (Valenciennes, 1828) |
Humpback grouper |
A |
DD |
H |
C, Ac |
|
|
138 |
|
|
Epinephelus areolatus (Forsskål, 1775) |
Areolate grouper |
B |
LC |
H |
C |
|
|
139 |
|
|
E. bleekeri (Vaillant, 1878) |
Dusky tail grouper |
B |
DD |
H |
C, Ac |
|
|
140 |
|
|
E. coioides (Hamilton, 1822) |
Orange-spotted grouper |
A, B, C, D |
LC |
H |
C, Ac |
|
|
141 |
|
|
E. latifasciatus (Temminck & Schlegel, 1842) |
Striped grouper |
B |
LC |
H |
C |
|
|
142 |
|
|
E. radiatus (Day, 1868) |
Oblique-banded grouper |
B |
LC |
H |
C |
|
|
143 |
|
Siganidae |
Siganus stellatus (Forsskål, 1775) |
Brown-spotted spinefoot |
A, B |
LC |
Vn |
C |
|
|
144 |
|
Sillaginidae |
Sillago sihama (Forsskål, 1775) |
Silver sillago |
A, B, C, D |
LC |
H |
C |
|
|
145 |
|
Soleidae |
Synaptura albomaculata Kaup, 1858 |
Kaup's sole |
A, B |
NE |
H |
C |
|
|
146 |
|
|
S. commersonnii (Lacepède, 1802) |
Commerson's sole |
A, B, C, D |
NE |
H |
C |
|
|
147 |
|
Sphyraenidae |
Sphyraena obtusata Cuvier, 1829 |
Barracuda |
A, B, C, D |
NE |
H |
C, Gf |
|
|
148 |
|
Stromateidae |
Pampus argenteus (Euphrasen, 1788) |
Silver pomfret |
A, B, C, D |
NE |
H |
C |
|
|
149 |
|
|
P. chinensis (Euphrasen, 1788) |
Chinese silver pomfret |
A, B, C, D |
NE |
H |
C |
|
|
150 |
|
Terapontidae |
Terapon jarbua (Forsskål, 1775) |
Jarbua terapon |
A, B, C, D |
LC |
H |
C |
|
|
151 |
|
|
T. puta Cuvier, 1829 |
Small-scaled terapon |
A, B |
NE |
H |
C |
|
|
152 |
|
|
T. theraps Cuvier, 1829 |
Large-scaled terapon |
A, B |
LC |
H |
C |
|
|
153 |
|
Trichiuridae |
Eupleurogrammus glossodon (Bleeker, 1860) |
Long tooth hairtail |
A, B, C, D |
NE |
H |
C |
|
|
154 |
|
|
E. muticus (Gray, 1831) |
Small head hairtail |
A, B, C, D |
NE |
H |
C |
|
|
155 |
|
|
Lepturacanthus savala (Cuvier, 1829) |
Savalai hairtail |
A, B, C, D |
NE |
H |
C |
|
|
156 |
|
|
L. pantului (Gupta, 1966) |
Coromandel hairtail |
A, B |
DD |
H |
C |
|
|
157 |
|
|
Trichiurus gangeticus Gupta, 1966 |
Ganges hairtail |
A, B, C, D |
NE |
H |
C |
|
|
158 |
Siluriformes |
Ariidae |
Arius arius (Hamilton, 1822) |
Threadfin sea catfish |
A, B, C, D |
NE |
Tr |
C |
|
|
159 |
|
|
A. jella Day, 1877 |
Blackfin sea catfish |
A, B, C, D |
NE |
Tr |
C |
|
|
160 |
|
|
A. maculatus (Thunberg, 1792) |
Spotted catfish |
A, B, C, D |
NE |
Tr |
C |
|
|
161 |
|
|
Nemapteryx caelata (Valenciennes, 1840) |
Engraved catfish |
A, B |
NE |
Tr |
C |
|
|
162 |
|
|
Plicofollis dussumieri (Valenciennes, 1840) |
Blacktip sea catfish |
B |
LC |
Tr |
Ss |
|
|
163 |
|
|
Sciades sona (Hamilton, 1822) |
Sona sea catfish |
B |
LC |
Tr |
C |
|
|
164 |
|
Plotosidae |
Plotosus canius Hamilton, 1822 |
Gray eel-catfish |
B |
NE |
Vn |
C |
|
|
165 |
Tetraodontiformes |
Diodontidae |
Diodon holocanthus Linnaeus, 1758 |
Long spined porcupinefish |
A, B |
LC |
Pn |
C, Aq |
|
|
166 |
|
Tetraodontidae |
Arothron hispidus (Linnaeus, 1758) |
White-spotted puffer |
A, B |
LC |
Pn |
C, Aq |
|
|
167 |
|
|
Chelonodon patoca (Hamilton, 1822) |
Milkspotted puffer |
A, B, C, D |
LC |
Pn |
C |
|
|
168 |
|
|
Lagocephalus guentheri MirandaRibeiro, 1915 |
Diamondback puffer |
A, B, C, D |
LC |
H |
Ss |
|
|
169 |
|
|
Takifugu oblongus (Bloch, 1786) |
Lattice blaasop |
A, B, C, D |
LC |
H |
Ss |
|
|
170 |
|
Triacanthidae |
Triacanthus biaculeatus (Bloch, 1786) |
Short-nosed tripodfish |
A, B |
NE |
H |
C |
|
|
171 |
|
Ostraciidae |
Tetrosomus gibbosus (Linnaeus, 1758) |
Humpback turretfish |
B |
LC |
Vn |
C, Aq |
|
Station: A = Machilipatnam; B = Nizampatnam; C = Vodarevu; D = Pakala. IUCN Status: VU = Vulnerable; LC = Least Concern; NE = Not Evaluated; DD = Data Deficient; NT = Near Threatened; EN=Endangered. Threat to Humans: Harmless = H; Traumatogenic = Tr; Venomous = Vn; Poisonous = Pn. Human Use: C = Commercial; F = Fisheries; Aq = Aquarium, Ss = Scientific study; Sf = Subsistence fisheries; Ac = Aquaculture; Gf = Gamefish; Ukn = Unknown.
Diversity Indices
The species diversity indices viz., i) Shannon-Wiener species diversity index (H’) (Shannon and Wiener, 1949), ii) Margalef richness index (SR) (Margalef, 1968) and iii) Simpson’s dominance index (D) (Simpson, 1949) were analyzed using the PAST (Palaeontological Statistics) software (Version 2.02).
Shannon-Weiner’s diversity index;
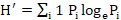
where, H’ = species diversity in bits of information per individual; Pi = ni/N (proportion of the samples belonging to the species; ni = number of individuals in all the samples; N = total number of individuals in the collection).
Margalef Species richness index;
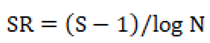
where, S = total number of species, and N = total number of individuals in the collection.
Simpson’s Dominance index;
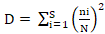
where, ni = number of individuals in the ‘each’ species, N = total number of individuals, S = total number of species.
RESULTS AND DISCUSSION
A comprehensive checklist of the species of fish recorded, the common names, site of occurrence at landing sites, IUCN status, risk to humans and usage are presented in Table 2. In the present study, a total of 171 species belonging to 14 orders, 63 families and 128 genera were recorded from four fish landing stations of Andhra Pradesh viz. Nizampatnam, Machilipatnam, Vodarevu, and Pakala. Of the total species, 17 species belonged to Chondrichthyes/Elasmobranchii, and 154 species belonged to Osteichthyes/Actinopterygii. The total number of species in various genera, families and orders recorded are presented in Table 3.
In India, mention may be made to the earlier works of Sudarsan (1988) which provided key to 273 species of fish in trawl catches off Visakhapatnam. Krishnan and Mishra (1993) reported 114 species from Kakinada, east coast of India. Sujatha (1995) reported 228 fish species belonging to 68 families from Visakhapatnam, Andhra Pradesh, southeast coast of India. Barman et al. (2004) documented 580 fish species under 292 genera, 121 families, and 27 orders from Andhra Pradesh. Gibinkumar et al. (2012) reported 191 fish species belonged to 12orders, 59 families and 109 genera from Cochin, southwest coast of India. Sambandamoorthy et al. (2015) reported123 fish species belonging to 13 orders, 49 families and 2 genera from the southeast coast of India.
The variation in the species number reported by different workers over a period of time could be due to the change in climate or the prevailing environmental conditions. In India, Andhra Pradesh is the second most cyclone-affected state (Babu et al., 2014; Bharti et al., 2017). During the study period, the impact of many cyclonic storms such as Titli, Gaja, and Phethai experienced on this coast might have prevented the fish from venturing into the sea resulting in less number of species recorded. The considerable decline might also be due to the reduction in the days of fishing due to cyclonic storms in Andhra Pradesh (FRAD, 2019).
Table 3: Number of Orders, Families, Genera and Species recorded in the present study.
| S. No | Order | Family | Genus | Species |
| 1 | Carcharhiniformes | 2 | 5 | 7 |
| 2 | Myliobatiformes | 2 | 4 | 5 |
| 3 | Orectolobiformes | 1 | 1 | 1 |
| 4 | Rhinopristiformes | 2 | 2 | 2 |
| 5 | Torpediniformes | 2 | 2 | 2 |
| 6 | Anguilliformes | 3 | 4 | 4 |
| 7 | Aulopiformes | 1 | 2 | 2 |
| 8 | Beloniformes | 3 | 4 | 4 |
| 9 | Clupeiformes | 4 | 13 | 22 |
| 10 | Elopiformes | 1 | 1 | 1 |
| 11 | Mugiliformes | 1 | 3 | 4 |
| 12 | Perciformes | 35 | 75 | 103 |
| 13 | Siluriformes | 2 | 5 | 7 |
| 14 | Tetraodontiformes | 4 | 7 | 7 |
| Total | 63 | 128 | 171 |
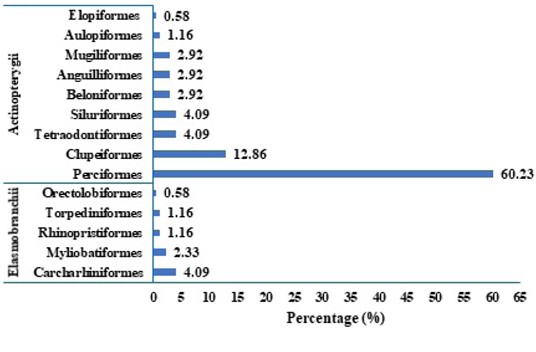
The relative percentage abundance of various orders of fish and the respective families, genera and species recorded from landing stations are depicted in Figures 3 and 4. The number of species recorded from various orders of fish is: Perciformes (60.23%) with 103 species, followed by Clupeiformes (12.86%) with 22 species, Siluriformes, Carcharhiniformes and Tetraodontiformes (4.09%) with 7 species each, Myliobatiformes (2.92%) with 5 species, Anguilliformes, Beloniformes, and Mugiliformes (2.33%) each with 4 species, Aulopiformes, Rhinopristiformes and Torpediniformes (1.16%) with 2 species each, and Elopiformes and Orectolobiformes (0.58%) with 1 species each. Among the orders, Perciformes is dominant (60.23%) representing 35 families with 103 species, a trend that is similar across various independent studies. Kar et al. (2017) reported 157 perciform species from coastal waters of West Bengal, and Jayaprabha et al. (2018) recorded 113 perciform species from Tamil Nadu, southeast coast of India.
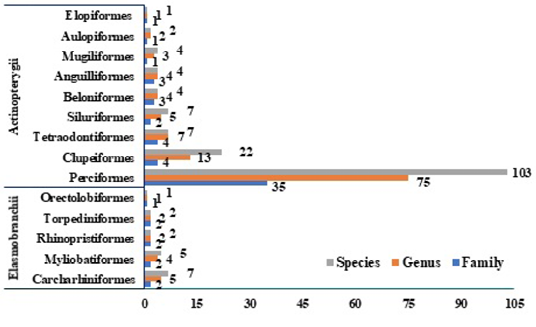
Figure 4: Relative Abundance of Species, Genera, Families and Orders of Fish recorded at four landing stations.
Table 4: Fish taxa in the four landing stations along coastal Andhra Pradesh.
| Taxa | Landing Stations | |||
| A | B | C | D | |
| Class | 2 | 2 | 2 | 2 |
| Order | 12 | 13 | 9 | 7 |
| Family | 51 | 59 | 31 | 30 |
| Genus | 106 | 118 | 69 | 67 |
| Species | 143 | 159 | 91 | 86 |
A = Machilipatnam; B = Nizampatnam; C = Vodarevu; D = Pakala
Considering the station-wise landing data, the number of species recorded at station B (159) and A (143) are relatively higher than those at stations C (91) and D (86) (Table 4). The majority of species numbering 73 (42%) were recorded in all four stations and in particular, 60 species (35%) were common in both A and B stations. This variation in species number may be due to the availability of mechanized trawl catches and fishing harbour facilities at stations A and B rather than at C and D. It is evident that among 171 species, commercial fishes are 153, and the remaining fishes are used for aquarium, aquaculture, fisheries, and sports and for research studies (Table 2).
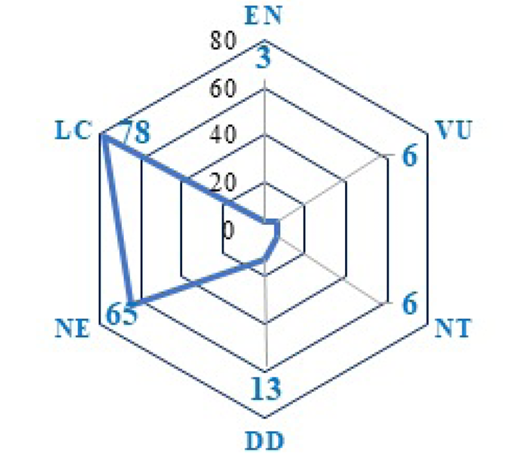
The Indian coastline contributes rich diverse fishery resources having substantial economic value (Darwin and Padmavathi, 2020). Despite commercial significance, no attempt has been made so far to know the conservation status for these fishes. According to IUCN red list of conservation status, among 171 fish species, 78 species (45.61%) are marked as Least Concern (LC), 65 species (38%) as Not Evaluated, 13 species (7.60%) as Data Deficient (DD) due to lack of adequate information, 6 species (3.5%) each as Near Threatened (NT) and Vulnerable (VU) and 3 species (1.75%) as Endangered (EN) (Figure 5). Out of 171 species, 65 species are not yet evaluated indicating an urgent need of conservation studies in these areas for establishing sustainable marine fisheries along the coast of Bay of Bengal. The conservation of fish also requires further studies on their complex life cycles.
Overfishing is one of the severe concerns affecting the community structure of fish with threatening and extinction of species (Jackson et al., 2001). Since the White cheek shark, Carcharhinus dussumieri, Broadfin Shark, Lamiopsis temminckii and Longheaded eagle ray, Aetobatus flagellum are recognized as Endangered (EN) species, immediate steps should be taken to stop catching these fish species for at least few years to rise their number to a reasonable level. Similarly, Blacktip shark Carcharhinus limbatus, Hammer head shark Sphyrna zygaena, Honeycomb stingray Himantura uarnak, Sharp nose stingray Maculabatis gerrardi, Giant guitarfish Rhynchobatus djiddensis, and Bigeye tuna Thunnus obesus are identified as Vulnerable (VU) fish species. Therefore, effective steps are essential to conserve the species and maintain harmony in the marine community and ensure sustainable management practice in the near future.
Different diversity indices were calculated with respect to four different sampling stations of coastal Andhra Pradesh. The values of Shannon-Wiener species diversity index (H’), and Margalef species richness index (SR) and Simpson dominance index value (D) were presented in Table 5. The maximum Shannon Diversity Index (5.069) was found at Nizampatnam station and the minimum (4.454) at Pakala. The highest Margalef species richness index (SR) of 31.17 was observed at Nizampatnam station whereas the lowest value of 19.08 at Pakala station. The maximum Simpson dominance value (D) of 0.994 was noticed at Nizampatnam and the minimum 0.988 in Pakala landing site.
Table 5: Fish Diversity Indices in Four Sampling Stations along Coastal Andhra Pradesh.
| Sampling Stations | Diversity indices | ||
| H’ | SR | D | |
| Machilipatnam | 4.963 | 28.61 | 0.993 |
| Nizampatnam | 5.069 | 31.17 | 0.994 |
| Vodarevu | 4.511 | 19.95 | 0.989 |
| Pakala | 4.454 | 19.08 | 0.988 |
H’ = diversity index; SR = species richness; D = dominance
Two main components involved in diversity of species are the species richness and distribution of individuals among species where the evaluation of species richness is complex (Williamson, 1973). In Shannon-Weiner index, the water and soil in aquatic environment has been considered as very poor quality when it is <1, poor quality 1-2, moderate quality 2-3, good quality 3-4. In the present study, the Shannon index was within the range of 5.069 - 4.454 which indicated that these study stations are in favorable conditions. A community becomes more divergent as the stress increases and consequently the species diversity decreases with unfavourable environmental conditions.A community with relatively few species represents that the environment is under stress (Plafkin et al., 1989). Species richness (SR) and Dominance (D) indices were found to be highest in Nizampatnam followed by Machilipatnam, Vodarevu and Pakala. The indices values were highest in Nizampatnam and Machilipatnam stations which indicate favourable conditions for fish abundance. When the temporal variation was compared, the species dominance among all the stations did not vary for a greater magnitude. The reason for more number of species at Nizampatnam and Machilipatnam stations might be due to the influx of estuarine species through Krishna river water to the marine fish stocks in between these landing stations. In addition to this, the ecological conditions have an effect on the distribution of fish species.
CONCLUSION
The present study provides information on marine fish diversity along the four landing stations of coastal Andhra Pradesh. It is evident that the recorded 171 species of fish upholds a vital fishery along the southeast coast of India. However, the species reported in the present study are lesser than those reported by Sudarsan (1981), Sujatha (1995), Barman et al. (2004), Gibinkumar et al. (2012), Kar et al. (2017). Most of the threats to the fish diversity in India are due to several natural and anthropogenic stress factors (Das et al., 2004; Gopi and Mishra, 2015; Joshi et al., 2015). The non-availability and less availability of some species indicate an alarming decline of marine fish diversity in the surveyed area and perhaps in the country as a whole. It is imperative to undertake a state-wide analysis of qualitative and quantitative availability of natural fish resources and the conservation status of marine fish. Therefore, a detailed long-term investigation of marine fish is needed to protect and conserve the species through effective policy decisions. This will pave the way for better conservation of natural fish diversity and benefit the mankind for more sustainable livelihood approach in the near future.
ACKNOWLEDGMENTS
The authors are thankful to the University Grants Commission (UGC), New Delhi, Government of India, for granting BSR (Basic Scientific Research) fellowship to Mr. Darwin Chatla and the authorities of Acharya Nagarjuna University for providing the necessary amenities to complete this work in the Department of Zoology & Aquaculture.
AUTHOR CONTRIBUTIONS
Both the authors contributed equally.
Conflict Of Interest
The authors have no conflicts of interest to the content of this article.
REFERENCES