Advances in Animal and Veterinary Sciences
Research Article
Immunogenicity and Antigenicity of Opisthorchis felineus Proteins
Vladimir Kiyan*, Aitbay Bulashev, Aibek Zhumalin, Ainura Smagulova, Ludmila Lider
Research Platform of Agricultural Biotechnology, Saken Seifullin Kazakh Agrotechnical University, Nur-Sultan, 010011, Kazakhstan.
Abstract | Three antigenic preparations of the human liver fluke Opisthorchis felineus were analyzed and characterized by immunochemical and serological reactions. The antigenic profiles of the excretory-secretory antigen (ES-Ag) contained 21 predominant protein bands in the range of 25-283 kDa, 20 protein bands in the range of 24-302 kDa of the somatic antigen (S-Ag), and 33 protein bands in the range of 23-313 kDa of the egg antigen. Stimulation of the immune system by these antigen preparations resulted in the production of specific mouse and hamster antibodies, which confirmed their immunogenicity. The antigenic characteristic of O. felineus antigens were measured by indirect-ELISA with specific sera. The average antibody titers when interacting with ES-Ag, S-Ag and E-Ag were in the range of 1:300, 1:3, 680 and 1:1,600, respectively. The 105-, 105- and 250-kDa protein bands of the ES-Ag, S-Ag and E-Ag preparations, respectively, were specific to positive serum from dogs infected with O. felineus. The activity and specificity of the antigen preparations in ELISA were positively correlated with dot immunoblot and Western blot assays.
Keywords | Opisthorchis felineus, Excretory-secretory antigen, Somatic antigen, Egg antigen, Immunogenicity
Received | June 17, 2020; Accepted | July 15, 2020; Published | August 01, 2020
*Correspondence | Vladimir Kiyan, Research Platform of Agricultural Biotechnology, Saken Seifullin Kazakh Agrotechnical University, 010011, Zhenis avenue, 62, Nur-Sultan, Kazakhstan; Email: [email protected]
Citation | Kiyan V, Bulashev A, Zhumalin A, Smagulova A, Lider L (2020). Immunogenicity and antigenicity of Opisthorchis felineus proteins. Adv. Anim. Vet. Sci. 8(9): 933-939.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.9.933.939
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Kiyan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Opisthorchis felineus (Rivolta, 1884) is the main liver fluke of the family Opisthorchiidae (Looss, 1899), which infects the liver of mammals and humans. O. felineus has been documented in humans and animals in 13 countries of the European Union (Pozio et al., 2013). This liver fluke also causes public health problems in the Russian Federation, Ukraine, Belarus and Kazakhstan (WHO, 1995). In Kazakhstan, this is the most common fluke species and is endemic in Pavlodar, Akmola, Karaganda, Northern Kazakhstan, Eastern Kazakhstan and Aktobe oblasts (Sultanov et al., 2014). O. felineus is considered responsible for serious diseases with high risks of cholangiocarcinoma emergence (IARC, 1994; Sripa et al., 2011; Armignacco et al., 2013; Chai et al., 2014; Fedorova et al., 2016). Early accurate diagnoses can prevent future problems and avoid health consequences.
Various techniques have been developed for identifying opisthorchiasis agents for accurate diagnoses. One such method, coproovoscopy, is laborious, time-consuming, requires qualified specialists, and fails to accurately identify opisthorchiid eggs. Serological test studies have suggested using antigens or antibodies specific to O. felineus for the diagnosis of opisthorchiasis (Teimoori et al., 2016; Bulashev et al., 2016; Waikagul et al., 2002; Starkova et al., 2011; Nöckler et al., 2003; Klebanovskaia, 1981, 1985).
Various components of parasites are used as antigens for immunodiagnostics and quantitative analyses of immune responses in infected hosts. These antigens can be classified as excretory-secretory, somatic and egg antigens (Sun et al., 1969; Kotelkin et al., 1997). Antigens of Opisthorchis have been suggested as target proteins for the serodiagnosis of opisthorchiasis. Opisthorchis antigens prepared from adult worms (somatic antigens) have molecular weights of 105, 90, 89, 63, 22.8, 16 and 15 kDa and can be used for immunodiagnoses (Wongratanacheewin et al., 2003; Glupov et al., 1997; Borovikov et al., 2010; Billings et al., 1990; Senawong et al., 2012). Excretory-secretory antigens have molecular weights of 28, 89 and 105 kDa specific to Opisthorchis (Bulashev et al., 2016; Kotelkin et al., 1997; Eursitthichai et al., 2010; Amornpunt et al., 1991). Egg antigens have been less studied but have been reported to contain immunogenic proteins of 66, 70, and 74 kDa (Kotelkin et al., 1997). Protein components of metacercariae, with molecular weights of 190-200, 132 and 107 kDa, react with opistorchiasis sera (Tanvanich et al., 1988). Among antigens of O. felineus, protein components of 15, 28, 63, 66, 70, 74, and 105 kDa have been reported as immunogenic proteins for serodiagnoses (Bulashev et al., 2016; Kotelkin et al., 1997; Glupov et al., 1997; Borovikov et al., 2010; Wongratanacheewin et al., 2003).
The comparative antigenicity and immunogenicity of O. felineus ES-Ag, S-Ag and E-Ag remain poorly understood, and available data in the literature are ambiguous and at times contradictory.
The aim of our study was to evaluate the serodiagnostic potential of these three groups of antigens to determine common immunoreactive proteins that can be used as antigens in ELISA for the diagnosis of opisthorchiasis at all stages of the disease.
Materials and Methods
Experimental animals
Fifty Syrian golden male hamsters (Mesocricetus auratus) (100-120 g body weight) at 8–10 weeks and twenty outbred laboratory male mice (Mus musculus L.) at 8–10 weeks (20-25 g body weight) were kept under good hygienic conditions at a vivarium of Saken Seifullin Kazakh Agrotechnical University (KATU). Their use and care were approved by the Animal Ethics Committee of Veterinary Medicine Faculty of KATU (Ethical approval letter, No.: 1, 09.11.2017) All activities involving animals were carried out according to Guidance for Accommodation and Care of Animals: Species-specific provisions for laboratory rodents and rabbits (Interstate Standard, GOST 33216-2014). The animals were provided with food and water ad libitum.
Obtaining O. felineus adult worms
Thirty hamsters were infected orally with 50 freshly isolated metacercariae. The infections were verified by coproovoscopy (Pavlyukov et al., 1991). On the 40th day post infection (p.i.), hamsters were euthanized with CO2, and adult trematodes were isolated from the livers to obtain antigens.
Preparation of excretory-secretory antigen (ES-Ag)
Adult trematodes were washed 4-5 times in PBS, pH 7.2-7.4, supplemented with penicillin (Simbirskaya Veterinary Company, Ulyanovsk, Russia) and streptomycin (Himfarm, Shymkent, Kazakhstan) (500 mg/ml), and immediately cultured. Viability was assessed by motility and general contractile movements. Obtained liver flukes were used to accumulate ES-Ag by cultivating in RPMI-1640 nutrient medium supplemented with 1 M HEPES, 200 mM L-glutamine, 100 mM sodium pyruvate (all from Sigma-Aldrich, St. Louis, USA), and 5,000 units of penicillin/streptomycin. Adult worms were cultivated as previously described (Bulashev et al., 2016, 2011) with some modifications. Briefly, 100 specimens of isolated O. felineus were cultivated per 15 ml during 5-6 days to accumulate maximum ES-Ag concentrations in nutrient medium. Cultivations were conducted at 37°C and 5% carbon dioxide. ES-Ag preparation included collection of culture supernatant, centrifugation at 15,000×g for 10 min, membrane filtration (cellulose acetate; 0,20 μm) and dialysis against PBS. Dialysis was performed using dialysis tubing cellulose membrane (Sigma-Aldrich, USA) with a pore diameter of 51 μm for 12 hours at 4 C. The protein concentration was determined by Bradford method (Bradford, 1976).
Preparation of somatic antigens (S-Ag)
S-Ag of O. felineus was prepared as previously described (Borovikov et al., 2010), with some modifications. Adult trematodes were washed several times in PBS (pH 7.2, containing 0.45% NaCl) and kept at -20°C for 18-24 hours (200 pieces per 1 ml of PBS solution). Adult worms were triturated after freezing by homogenizing (mechanically) and centrifuged at 3,000×g for 30 min. Supernatant was collected and proteins were precipitated with 30% ammonium sulfate at 6-8°C for 12 hours. The precipitate (S-Ag fraction 1) was separated by centrifugation (3,000×g for 30 min) and dissolved in 2 ml of 0.05 M Tris-HCl buffer (pH 8.2) containing 0.5 M NaCl. The protein concentration was determined by Bradford method (Bradford, 1976).
The remaining pellet was subjected to full decomposition by dissolving in 0.5 ml lysis buffer containing 20 mМ Tris-HCl (pH7.5), 150 mМ NaCl, 1 mM EDTA, 1 mM EGTA, 0.5% Triton X-100, 0.3% C24H39NaO4, 2.5 mM Na4P2O7, 2.5 mМ β-glycerophosphate and protease inhibitor cocktail (Roche, Mannheim Germany), sonicating (amplitude 40, at 4°C for 30 seconds, 2 times) and pelleting at 21,000×g for 10 mins. The supernatant (S-Ag fraction 2) was analyzed for total protein composition. The resulting preparations (S-Ag fraction 1 and 2) were used as somatic antigens.
Preparation of egg antigen (E-Ag)
To prepare the egg antigen, marites were cultured in a nutrient medium as described above. After 24 hours, the culture medium was analyzed under a microscope for the presence of egg release in sexually mature marites. The culture medium with eggs was centrifuged at 1,000×g for 5 min, and the supernatant was saved for ES-Ag purification. The egg pellet was collected, washed twice in PBS and used for antigen preparation. Briefly, 0.5 ml of lysis buffer was added to the pellet, then sonicated (amplitude 40, at 4°C for 30 seconds, 2 times) and centrifuged at 21,000×g for 10 min. The resulting supernatant was used as an egg antigen.
The immunization of laboratory animals
The immunogenicity of O. felineus antigens was studied by immunizing hamsters and outbred mice. Five outbred mice were given intraperitoneal (i.p.) injections of 0.1 mg of the ES-Ag, S-Ag or E-Ag antigen in 0.1 ml of complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, USA) on day 0. The appropriate antigen was emulsified in Freund’s incomplete adjuvant (Sigma-Aldrich, St. Louis, USA) and injected i.p. in an amount of 0.2 ml on day 7. Subsequent injections of antigen in 0.1 ml of phosphate-buffered saline (PBS), pH 7.2–7.4, (Amresco, Solon, USA) were performed i.p. on days 11, 12 and 13. Serum samples were taken on days 16 and 31 to study immunogenicity of O. felineus ES-Ag, S-Ag and E-Ag from outbred mice. Blood was centrifuged at 1,000×g for 10 min, and serum was transferred into a clean tube. Fifteen hamsters were immunized with these antigens according to the same scheme, but the concentration of injected antigens was 0.2 mg.
Canine/dog serum samples
The serum samples of 6 experimentally infected dogs were collected during our previously study and were stored at −70°C until use (Kiyan et al., 2016). Fife dogs were infected by feeding skinned fish fillets containing metacercariae of O. felineus every 5–7 days over the course of two months and one dog served as control. The infection of dogs was carried out without taking into account the number of larvae. From three weeks post infection onward, stool samples from each dog were collected weekly for microscopic examination by conventional Fülleborn’s method (Fülleborn, 1920). Serum samples were taken every seven days after infection.
Indirect ELISA for the detection of antibodies against O. felineus antigens (ES-Ag, S-Ag and E-Ag)
A microtiter plate was filled with antigen (5.0 μg/ml) in PBS (pH 7.2) and incubated at 4°C overnight. After washing 3 times with PBS-T, the active centers of the wells were neutralized with 1% BSA, serum samples were added and the plate was incubated for 1 h at 37°C. Nonspecific components were removed by washing with PBS-T. The presence of specific antibodies was detected using peroxidase-conjugated goat anti-mouse IgG (H+L) (Jackson Immuno Research, West Grove, USA) or peroxidase-conjugated goat anti-hamster IgG (H+L) (Sigma-Aldrich) and its substrate. As a control negative serum, a sample taken from mice and/or hamsters on day 0 before first immunization was used. ELISA results were considered positive if the OD value of the well with test serum was higher by at least twice the OD value of the control well. Analysis was performed in triplicate.
Statistical analysis was performed to determine the significance of differences between antibody titres (Sayduldin, 1992).
SDS-PAGE and Western blot
A 4-12% SDS-PAGE gradient gel (BioRad, California, USA) was performed as described by (Laemmli, 1970). Proteins of O. felineus antigens were visualized by Coomassie blue staining or Silver Quest Staining Kit (Invitrogen, Waltham, Massachusetts, USA). The molecular masses of the protein bands were determined using Photo-Capt, Version 12.4 (Vilber Lourmat, France). After electrophoresis, proteins were transferred onto a PVDF membrane (Millipore, Darmstadt, Germany), and immunoblotting was carried out as described by (Towbin et al., 1979). After blocking in a solution of 5% bovine serum albumin (BSA) at room temperature for 1 h, the PVDF membrane was incubated with specific antibodies at room temperature for 2 h. HRP-conjugated goat anti-dog IgG-heavy and light chains (Bethyl Laboratories, Inc., Montgomery, TX, USA) were used at 1:10,000 dilution in phosphate buffered saline with Tween-20 (PBS-T) at room temperature for 1 h for detection of antigen-antibody complexes. Complexes were detected by enhanced chemoluminescence (ECL) using an Immobilon Western kit (Millipore, Darmstadt, Germany).
Immunodot blot analysis
Immunodot blot analysis was conducted using a nitrocellulose membrane (Bio-Rad, Hercules, California, USA) on which various antigen dilutions in the form of dots were fixed (1:2-1:1,024). The nitrocellulose membranes were left at room temperature for 30 min to allow antigen immobilization. The dots were subsequently blocked for an hour with 5% skim milk in PBS containing 0.05% Tween-20 and then washed three times with PBS-T. Thereafter, the membranes were incubated for 2 h with dog’s sera diluted 1:100 in 5% skim milk–PBS and washed three times with PBS-T. This was followed by addition of HRP-goat anti-dog IgG-heavy and light chains diluted 1:30,000 in 5% skim milk–PBS. The subsequent washing steps and detection procedures were performed according to the ECL Plus manual. All analyses of serum samples were performed in triplicate.
Results
Obtaining of O. felineus
Hamster infections were monitored after 30 days i.p. by coproovoscopy. Parasites eggs found in the feces of the infected hamsters indicated the presence of adult worms. At post-mortem examination on the 40th day i.p., adult samples of O. felineus (based on the morphological features) were obtained from bile ducts and gall bladders of thirty infected animals with an infection intensity 16.3±3.9 flukes.
Preparation and electrophoretic study of antigens
The final concentration of O. felineus ES-Ag in the RPMI-1640 nutrient medium on the 5th day of cultivation was in the range of 0.5-1.0 mg/ml. The concentration of S-Ag was 1.5 mg/ml and that of E-Ag was 1.0 mg/ml.
Protein analysis by SDS-PAGE indicated the presence of 21 predominant protein bands in the structure of O. felineus ES-Ag (25-283 kDa molecular weight (m.w.)), 20 protein bands in the structure S-Ag (24-302 kDa) and 33 protein bands in the E-Ag (23-313 kDa; Figure 1).
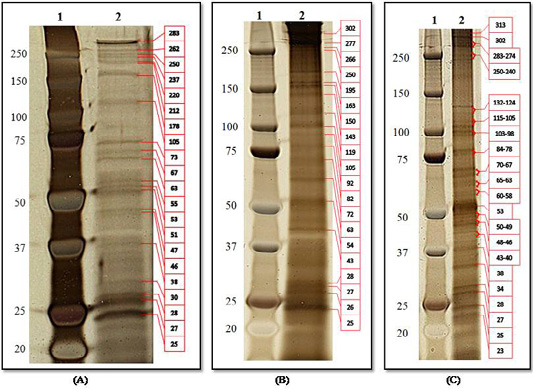
Figure 1: Protein analysis of O. felineus antigens by SDS-PAGE and visualization by Silver Quest Staining Kit: (A) ES-Ag; (B) S-Ag; (C) E-Ag. Line 1, molecular markers; line 2, electropherogram of antigens.
The dominant proteins for the three types of antigens had m.w. of 25, 27, 28, 63, 105 and 250 kDa. Additionally, the same proteins of ES-Ag and E-Ag had m.w. of 38, 46, 53, 67 and 283 kDa. For S-Ag and E-Ag, same proteins had a m.w. of 43 kDa.
Immunogenicity of O. felineus antigens
On day 16, the average antibody titers in sera of mice and hamsters immunized with ES-Ag were 1:260 and 1:1,060 (t=1.84, P<0.2), respectively (Table 1).
Table 1: Immunogenicity of O. felineus antigens in mice and hamsters by indirect-ELISA.
| Antigens | Antibody titers against O. felineus antigens in animals groups | |
| Outbred mice (n=5) | Syrian golden hamsters (n=5) | |
|
ES-Ag |
1:260 (+70.5; -41.3) |
1:1060 (+70.5; -41.3) |
|
S-Ag |
1:530 (+48.5; -32.7) | 1:1390 (+49.5; -33.0) |
|
E-Ag |
1:700 (+30; -23.1) | 1:1600 (+30.1; -23.1) |
The stimulation of the immune system by S-Ag resulted in production titer yields of specific antibodies of mice and hamsters of 1:530 and 1:1,390 (t=1.12, P<0.15). The mean titers of mice and hamsters immunized with E-Ag during this period increased significantly and were 1:700 and 1:1,600 (t=2.21, P<0.1), respectively. Thus, the studied antigens were able to induce immune responses in the form of antibody formation, which confirmed their immunogenicity.
Antigenicity of O. felineus antigens
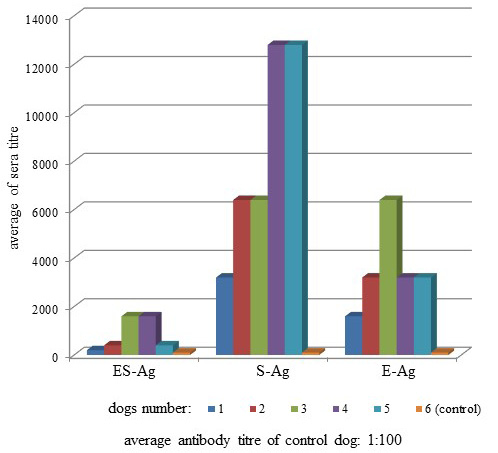
Antibodies of the infected dogs were specific to O. felineus antigens (Figure 2). The average antibody titers in the sera of infected dogs when interacting with ES-Ag, S-Ag and E-Ag were 1:300 (+48.5; -32.7), 1:3,680 (+30.1; -23.1) and 1:1,600 (+30.1; -23.1), respectively.
O. felineus antigens in dog sera with strong ELISA activity were further characterized by dot immunoblot and Western blot assays (Figure 3).
The ES-Ag dilutions interacting with positive sera were 1:8 to 1:32 (Figure 3A). The use in the dot immunoblot assays of S-Ag dilutions and same positive sera demonstrated better results and were 1:128 to 1:512 (Figure 3B). The E-Ag dilutions under the same reaction conditions showed good results and were 1:64 to 1:256 (Figure 3C).

Figure 3: Dot immunoblot patterns of O. felineus antigens in sera of infected and control dogs: (A) ES-Ag; (B) S-Ag; (C) E-Ag. Line 1, 2, 3, 4, 5, sera of infected dogs; line 6, sera of the control dog. The numbers on the left indicate to antigen dilutions that reacted with serum.
Western blots with the ES-Ag and positive sera from 3 and 4 infected dogs yielded a single discrete band at the 105-kDa position. However, with the S-Ag and E-Ag preparations, the polyclonal antibodies reacted with two protein components that migrated at the 105 and 250-kDa positions (Figure 4).
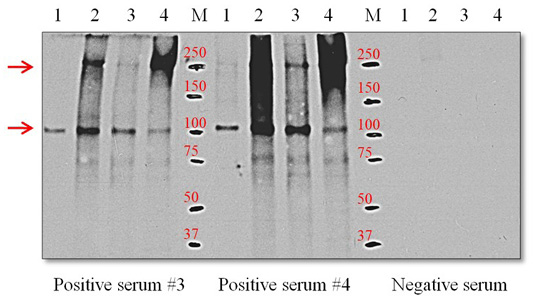
Figure 4: Western blot using positive serum with O. felineus antigens. Line 1, ES-Ag; line 2, S-Ag fraction 1; line 3, S-Ag fraction 2; line 4, E-Ag; M, molecular markers. Molecular mass markers are expressed in kDa. The arrows on the left indicate proteins with molecular masses of 105 and 205 kDa that react with positive serum.
Discussion
In Kazakhstan, the diagnosis of opisthorchiasis is made on the basis of the detection of Opisthorchis eggs in feces and duodenal contents. The results of serological methods of research (ELISA) are not the basis for diagnosis because there are additional diagnostic studies and indirect signs of opisthorchiasis invasion (Order No. 283, 2015). This is because the available immunological tests for detecting specific Opisthorchis antibodies are not satisfactory. Applicable serological diagnoses of O. felineus are an unmet need (Bulashev et al., 2016; Waikagul et al., 2002; Starkova et al., 2011; Kotelkin et al., 1997; Glupov et al., 1997; Borovikov et al., 2010).
The results presented in this study show that the three type preparations obtained from O. felineus can be used as antigens for immunodiagnosis. Recent proteomic analyses of excretory-secretory products of O. felineus showed complex protein compositions. Thirty-seven proteins in ES-Ag O. felineus have been identified by high-resolution proteomics; such an approach could be useful for identifying proteins that cannot be detected by immunoblotting because of their low content in comparison to major parasite protein components (L’vova et al., 2014). However, no data exist in the literature for proteomic studies of somatic and egg antigens. In this regard, it is important to isolate immunogenic proteins specific for O. felineus from antigenic preparations as potential antigens for vaccine and immunodiagnostic development.
Our results showed that we identified more proteins in antigens than other studies. First, this was because we used a 4-12% SDS-PAGE gradient gel to separate the antigens, which is permeable to high m.w. protein components (Figure 1). Second, the Silver Quest Staining Kit used to color the SDS-PAGE gel has a higher resolution than the Coomassie blue method.
All three types of antigens elicited strong immune responses in laboratory animals. E-Ag has the highest immunogenicity; the antibody titers of laboratory animals immunized with E-Ag were higher than the ES-Ag and S-Ag antigens. This was observed in two types of laboratory animals. Hamster immune status was more sensitive to O. felineus antigens than the mice; specific antibody titers in the blood serum after immunization were much higher in all experimental groups (Table 1).
Antibodies of the infected dogs in the indirect-ELISA were specific to all prepared antigenic preparations of Opisthorchis felineus. Moreover, according to the titers of specific antibodies, S-Ag was more active than ES-Ag and E-Ag by its antigenicity (Figure 2). The present study also showed that the three types of antigens interacted in different ways with the sera of the infected dogs because of substantial differences between titers of specific antibodies. Thus, when we used ES-Ag, the highest titers showed 3 and 4 sera (1:1,600), S-Ag interacted better with 4 and 5 sera (1:12,800), and the highest antibody titers showed serum 3 in the interaction with E-Ag (1:6400). Similar data were observed from the study of antigen activity by dot immunoblot assay. S-Ag was also more active than ES-Ag and E-Ag by its antigenicity as shown in Figure 3.
E-Ag was the most immunogenic in the laboratory animals, but less antigenic with the sera of the infected dogs. This was because after the dogs were infected, parasite egg secretions only began after 30 days i.p. Thus, the dog immune response only began later to respond to the eggs and not to the parasite itself.
The results presented in Figure 4 show that the positive sera obtained from infected dogs with the ES-Ag antigen of O. felineus recognized the immunoreactive 105-kDa protein component of the parasite. This immunoreactive 105-kDa protein component is also present in the S-Ag and E-Ag antigens. Previous studies have also reported on the immunogenicity of this 105-kDa protein and the possibility of its use in diagnoses (Kotelkin et al., 1997; Glupov et al., 1997). For the first time, we described an immunoreactive 250-kDa protein component in the S-Ag and E-Ag antigens. It should be noted that 250-kDa protein component is more antigenic than the 105-kDa protein component. This is clearly illustrated in Figure 4. The presence of two dominant immunoreactive proteins in the S-Ag and E-Ag antigens explains their high immunogenic (Table 1) and antigenic activity (Figures 2 and 3). Proteins with the lowest molecular weights in the antigens have relatively large proportions of the total composition of proteins but do not possess pronounced immunogenicity.
Conclusion
Opisthorchis felineus ES-Ag, S-Ag and E-Ag contain a common immunoreactive protein with a m.w. of 105 kDa that can be used for the serological diagnosis of opisthorchiasis at all stages. However, further studies are necessary to determine the sequence of this immunoreactive protein and its production by recombinant DNA technology. The use of a recombinant antigen for the diagnosis of opisthorchiasis is advantageous because the antigen can then be produced at preparative scales to develop more convenient and inexpensive serological assays.
Acknowledgements
The authors are grateful to Orken S. Akibekov, Sadibek S. Tokpan and Zhasulan K. Baibolin (Saken Seifullin Kazakh Agrotechnical University, Nur-Sultan, Kazakhstan) for their technical support during the research activity. This investigation was financially supported by the Ministry of Education and Science of the Republic of Kazakhstan in the frame of the projects No. AP05131132 for 2018–2020.
Authors Contribution
All authors shared equally in designing, conducting the study and writing the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References