Advances in Animal and Veterinary Sciences
Research Article
Clinical Study with Investigation of Polymorphism FSHR Gene in Twining Goats in Iraq
Saad Akram Hatif*, Hayder Abdul-Kareem AL-Mutar, Khawla A Hussain
College of Veterinary Medicine, Baghdad University, Iraq.
Abstract | This study included the insemination of 15 fertile local Iraqi goats. The experimental goats were examined by abdominal palpation at 45, 60 and 90 days, and were performed ultrasound to confirm the presence or absence of the pregnancy at 30, 35, 50, and 55 days post-insemination. Ultrasonographic examination was performed using prop 3.5MHz for abdominal examination with special gel for the prop. The study aimed to investigate the polymorphism of FSHR gene, which is responsible of fertility by reaction with the FSH hormone. The results revealed that the twining pregnancy was detected in 10 goats while the other 5 goats have single kids. The polymorphism was identified at three cleavage sites (304 bp, 214 bp, and 90 bp) within the amplification fragment. Three genotypes BB (304 bp) twin birth, AA (214bp/90 bp) single birth, and AB (304 bp/214 bp/90bp) twin birth were detected in local Iraqi goat.
Keywords | FSHR gene, Polymorphism, Twin, Iraqi goats.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | February 28, 2017; Accepted | April 12, 2017; Published | April 29, 2017
*Correspondence | Saad Akram Hatif, College of Veterinary Medicine, Baghdad University, Iraq; Email: [email protected]
Citation | Hatif SA, Al-Mutar HAK, Hussain KA (2017). Clinical study with investigation of polymorphism fshr gene in twining goats in Iraq. Adv. Anim. Vet. Sci. 5(5): 192-195.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.5.192.195
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Hatif et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The function of follicular stimulation hormone (FSH) relate with the FSH receptor (Tisdall et al., 1995). Follicle stimulating hormone has many functions in granulosa cells, including proliferation and differentiation effects. The diverse responses of granulosa cells to FSH during follicle growth (Wayne et al., 2007) are likely a consequence of the receptor to which FSH binds (Sairam and Babu, 2007). FSHR is found on granulosa cells of developing follicles. The binding of FSH to the FSHR stimulates granulosa cell division and steroidogenesis. The chromosome 2, including the gene expression to the receptor FSHR gene provided 10 exons. The first 9 exons encoded to extracellular domain and the transmembrane and intracellular domain encoded by the last one (Segaloff and Ascoli, 1993). The major components of the hypothalamic pituitary- gonad axis in the corresponding with the FSH and LH that is responsible of the performance of the function of reproduction and the production of gametes and fertility. Glycolytic enzymes in oocytes which is regulating expression levels of genes is control at glycolysis in granulosa cells (Sugiura et al., 2005).
The study aimed to investigate the twin pregnancy by using ultrasonography and to Detect the polymorphismof FSHR gene by PCR-RFLP in twining goats.
MATERIALS AND METHODS
A total of 15 Iraqi goats ranged of 2.5-4 years old with history of single and twining birth were used. The females were diagnosed by abdominal palpation at 45, 60, and 90 days and subjected to ultrasonography for confirmation of the pregnancy at 30, 35, 50, and 55 days post-insemination. Ultrasonography examination was performed using prop 3.5MHz for abdominal examination with specialized gel.The ventro-lateral area of the abdomen was cleaned, clipped and shaved for abdominal examination. The animals were setting, and casting on ground with the maintenance at the four limbs to left up and controlled by another person .Blood sample (5 ml) was gatheredaseptically from jugular vein of goat into tubes containing anticoagulants (0.5 M EDTA) for each animal. The total DNA produced by the standard protocol by intron kit (Korea) procedure. Two conserved primers, forward: 5- CCCATCTTTGGCATCAGC-3 and reverse: 5- ACACAGTGATGAGGGGCAC-3. Thermal cycling included : Denaturation at 95 °C for 3 min, followed by 35 cycles of 94 °C for 30s, 55°C for 30s and 72 °C for 35s with final incubation at 72 °C for 7 min. Polymerase chain reaction products were extracted using electrophoresis at 2% agarose gel with the visualized by contact with ultra violet light (302nm). Sequence of nucleotides of exon 10 of FSHR gene by using BioEdit program, that is performed in the National Instrumentation Center for Environmental Management (NICEM) online at, DNA sequencer 3730XL, Applied Biosystem. Chi square used for statistical analysis (SAS, 2012).
RESULTS
This study conducted on 15 local Iraqi goats that were inseminated, followed by pregnancy diagnosis using 3.5MHz probe. The results of ultrasonography diagnosis in early stage of pregnancy 30, 35, 50 and 55 day detected some goats were pregnant with twin embryos while another with single embryo (Figure 1). Tengoats have twin and 5 goats have single embryo. It’s very obvious and clear image of uterine content. Molecular examination involves the genomic DNA extraction from 10 blood samples of goats by intron kit , polymerase chain reaction for gene visualized by electrophoresis on the band size 304 bp (Figure 2).
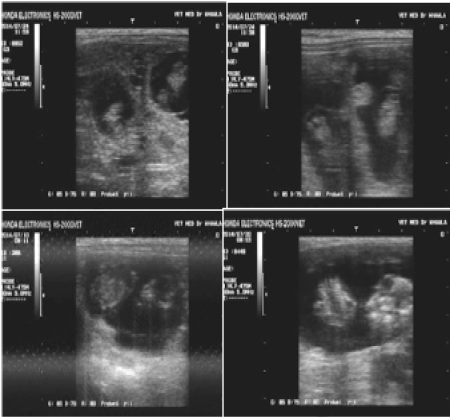
Figure 1: Pregnancy diagnosis of goats by ultrasonography : a. twin in 30 days, b. twin in 35 days, c. single in 50 days, and d. single in 55 days
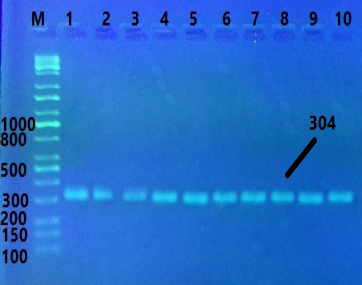
Figure 2: The product was electrophoresis on 2% agarose gel at 5 volt/cm2, 1x TBE buffer for 2 hours. M: DNA ladder (100-10000bp), Lane 1-10 product for FSHR gene of Goat, PCR product of band size 304 bp. visualized under U.V light after staining with red stain safe
The PCR product was digested with restriction endonucleases and the genetic polymorphisms were investigated by PCR-RFL. There are three cleavage sites (304 bp, 214 bp, and 90 bp) within the amplification fragment. Three genotypes BB (304 bp), AA (214bp/90 bp) and AB (304 bp/214 bp/90bp) were detected in local Iraqi goat (Figure 3) and (Table 1).
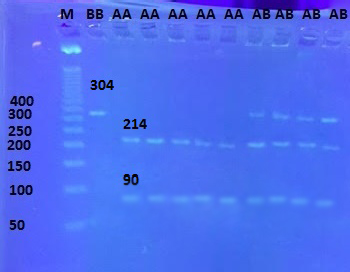
Figure 3: Electrophoresis pattern of PCR product digested with restriction enzyme (2% agarose gel) in goat. Lane’s 2,3,4,5,6 homozygous: AA genotype (single birth); Lane’s 7,8,9,10 heterozygous: AB genotype(twin birth) . Lane’s 1 homozygous: BB genotype (twin birth) with. M: DNA molecular marker 100 bp size .by red stain in the gel
Amounts of repeat alleles A and B that returned to exon10 FSHR gene in the present study on Iraqi does were 0.93 and 0.7, respectively (Table 2).
Table 1: Distribution of gene polymorphism in single and twin Iraq goat.
|
Genotype |
No. of animal (15) |
Percentage (%) |
||
|
Single 5 |
Twin 10 |
|||
|
AB |
0 |
4 |
0% |
40% |
|
AA |
5 |
5 |
50% |
50% |
|
BB |
0 |
1 |
0% |
10% |
|
Total |
||||
Table 2: Allele frequency of FSHR gene in sample study
|
Allele |
Allele frequency |
|
A |
0.93 |
|
B |
0.7 |
|
Total |
1 (100%) |
Discussion
The results indicated that it is sufficient for pregnancy diagnosis. The females were diagnosed by abdominal palpation at 45, 60, and 90 days. Pregnancy diagnosis of inseminated goat included abdominal palpation and abdominal palpation considered a very important tool in pregnancy diagnosis (Travenes and Noakes, 2009). Abdominal was subjected by ultrasonography for confirmation of the pregnancy at 30, 35, 50, and 55 days post-insemination. Pregnancy diagnosis in goat in early period at 26 days by abdominal transducer at 3.5 MHz transducer (Padilla-Rivas et al., 2005), and The embryonic vesicles were detected at days 14 (Mendan et al., 2004). The PCR product of FSHR gene was electrophoresis. The variant forms of the FSHR are possibly expressed differently by granulosa cells at different stages of follicular growth (Sairam and Babu, 2007).The function and the differentiation of granulosa cells controlled by oocyte that effect on the gene expression in the follicular somatic cells (Sugiura and Eppig, 2005). In order to investigate the polymorphism in the FSHR gene and to assess the percent of single and twining in the goats.The reproductive performance related with the FSHR gene polymorphisms in goat (Xiaohong et al., 2013). FSHR gene SNPs were analyzed by PCR and RFLP (Livshyts et al., 2009) The polymorphism in the FSHR in goat in Iraq related with the source variant of the origin of goat in Iraq. In the present study, the primers for the exon 10 of goat FSHR gene were used for amplification genomic DNA of local goats and a uniform fragment of 304 bp was obtained after 2% agarose gel electrophoresis in local goat. The variants in FSHR gene were proved to be associated with reproductive diseases in human such as polycystic ovary syndrome (PCOS) (Gu et al., 2010).Three genotypes BB (304 bp), AA (214bp/90 bp) and AB (304 bp/214 bp/90bp) were detected in local Iraqi goat. Five mutations mentioned above had significant effect on the litter size in Guizhou Black goats (P < 0.05), and the litter size of does with genotype BB was significantly higher than that of does with genotypes AA and AB (Xiaohong et al., 2013). The genomic environment surrounding FSHR is relatively devoid of other coding genes, with its closest neighbor Lhcgr over 200 kb away, placing it in a class of genes that reside in gene deserts (Nobrega et al., 2003; Ovcharenko et al., 2005). This study investigate the mutation of goal gene that effect of sone fertility in the Iraqi goats. FSHR inactivating mutations may cause infertility and premature ovarian failure (POF), whereas activating mutations can predispose to ovarian hyper stimulation syndrome (OHSS) as a consequence of exogenous FSH administration (Lussiana et al., 2008).
CONCLUSION
In conclusion there are three genotypes that were detected in local Iraqi goats and are distributed in twining and single pregnancy.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this research paper.
Acknowledgements
Deep gratitude should go to all members of Baghdad agriculture research center, for their help. I would like to thank the team of Biotechnology Research Center, Al-Nahrain University.
Authors’ Contributions
All authors contributed equally.
REFERENCES






