Advances in Animal and Veterinary Sciences
Research Article
Prevalence of Canine Babesiosis in Different Breeds of Dogs in and Around Bengaluru
Mohan Kumar Chillahalli Mahalingaiah1, Muralidhara Asoor2, Ramesh Poojari Thimmaiah1, Hogalagere Doddappaiah Narayanaswamy3, Shivalingappa Yamanappa Mukartal4, Anuradha Menon Elattuvalappil5, Nishanth Chikkahonnaiah6, Saurabh Gupta7, Shoorvir Singh7
1Department of Veterinary Medicine, Veterinary College, Hebbal, Karnataka Veterinary Animal and Fisheries Sciences University(KVAFSU), Bengaluru-560024 ; 2Department of TVCC,Veterinary College, Hebbal, Karnataka Veterinary Animal and Fisheries Sciences University(KVAFSU), Bengaluru-24; 3Department of Pathology, Veterinary College, Hebbal, Karnataka Veterinary Animal and Fisheries Sciences University(KVAFSU), Bengaluru-560024; 4Department of Veterinary Microbiology, Veterinary College, Hebbal, Karnataka Veterinary Animal and Fisheries Sciences University(KVAFSU), Bengaluru-560024 ; 5ICAR NAE project, Veterinary College, Karnataka Veterinary Animal and Fisheries Sciences University(KVAFSU), Bengaluru-560024; 6Department of Veterinary public health and epidemiology, Veterinary College, Hebbal, Karnataka Veterinary Animal and Fisheries Sciences University (KVAFSU), Bengaluru-560024.7Microbiology Laboratory, Animal Health Division, Central Institute for Research on Goats, Makhdoom, PO - Farah, Mathura, 281122, Uttar Pradesh.
Abstract | Canine babesiosis is a tick borne hemolytic disease caused by intra-erythrocytic protozoan belonging to genus Babesia, comprising two main species B. canis and B. gibsoni.Present study estimated the prevalence of canine babesiosis in dog population located in and around Bengaluru city of Karnataka state. Prevalence was estimated on the basis of history, clinical signs and detectionof the organisms in the blood of infected dogs. Dogs (>70.0%) exhibited clinical symptoms of high fever (104°F), anemia, lethargy, history of tick infestation, congested/pale/icteric mucous membranes, hematuria and epistaxis.A total of 102 blood samples from dogs suspected for canine babesiosis were collected from different breeds, gender and age group of animals and presented to Veterinary College Hospital, Hebbal, Bengaluru. Of 102 samples screened, 40 (39.2%) and 66 (64.7%) were positive by blood microscopy and PCR, respectively. Overall prevalence of canine babesiosis was 31(30.3%)and 50(49.0%) for B. canis and B. gibsoni, respectively. Mixed infections both with B. canis and B. gibsoni were detected in 25 (24.50 %) samples. Higher incidence of canine babesiosis was seen in the age group above 1-2 years (23%), breed-wise in Labrador Retrievers (26.0%) and gender-wise in male dogs (57.5%). Prevalence of canine babesiosis was moderately high in dogs. Adoption of combination of blood microscopy as screening test and PCR as confirmatory test,as a strategy for diagnosis of canine babesiosis in dogs is recommended.
Keywords | B. canis, B. gibsoni, PCR, Blood microscopy, Canine babesiosis.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | December 11, 2016; Accepted | January 26, 2017; Published | March 20, 2017
*Correspondence | Shoorvir Singh, Microbiology Laboratory, Animal Health Division, Central Institute for Research on Goats, Makhdoom, PO - Farah, Mathura, 281122, Uttar Pradesh; Email: [email protected]
Citation | Mahalingaiah MKC, Asoor M, Thimmaiah RP, Narayanaswamy HD, Mukartal SY, Elattuvalappil AM, Chikkahonnaiah N, Gupta S , Singh S (2017). Prevalence of canine babesiosis in different breeds of dogs in and around bengaluru. Adv. Anim. Vet. Sci. 5(3): 140-144.
DOI | http://dx.doi.org/10.14737/journal.aavs/2017/5.3.140.144
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Mahalingaiah et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Amongst the various prevalent canine vector-borne diseases, canine babesiosis is a clinically significant disease caused by intra-erythrocytic protozoa belonging to genus Babesia, which has beenreported worldwide including India (Patton, 1910; Kjemtrup and Conrad, 2006). Babesia canis (large 3.0–5.0 μm) and B. gibsoni (small 1.5–2.5 μm) are recognized as two species which causes canine babesiosis worldwide (Jefferies et al., 2003). Babesia infection is transmitted by ticks Dermacentor reticulatus, Rhipicephalus sanguineus, Haemaphysalis leachi. The tick vector is usually specific to each species of Babesia. The occurrence of Babesia spp. in dogs and their geographical distribution are related to the distribution of their vector species (Solano-Gallego and Baneth, 2011). Transmission can also occur from dog to dog through bite (Jefferies et al., 2007) and transplacental transmission (Fukumoto et al., 2005).
Clinically canine babesiosis has been found to result in a wide range of presentations from sub clinical to serious illness characterised by fever, depression, pallor, jaundice, lymphadenopathy, splenomegaly, weakness and collapse associated with intravascular and extravascular haemolysis, hypoxic injury, systemic inflammation, thrombocytopenia. After initial acute infection, the animal may become a chronic carrier (Irwin, 2009).
Currently definite diagnosis of canine babesiosis is based on haematological, biochemical results and demonstration of the parasite by light microscopy. Various serological methods such as complement fixation test (CFT), immunofluorescent antibody test and enzyme linked immunosorbent assay (ELISA) were used. But the polymerase chain reaction (PCR) assay present a higher sensitivity and specificity than the blood smear evaluation and may differentiate species that may not be morphologically distinguished by smear method (Boozer and Macintire, 2003). Information does not exist on the prevalence of canine babesiosis in dogs in and around Bengaluru.Hence present study was undertaken to estimate the prevalence of canine babesiosis in dogs based on age, breed and gender in and around Bengaluru.
MATERIALS AND METHODS
Collection of Samples and Tests
A total of 102 blood samples were collected from dogs with clinical signs of high fever of 104°F, inappetance, anemia, lethargy, history of tick infestation, congested/pale/icteric mucous membranes, lymphadenopathy, hematuria and epistaxis from different breeds, gender and age group of 1 - 11 years, which were presented to Veterinary College Hospital, Hebbal, Bengaluru. Blood samples were collected in vacutainer containing EDTA as anticoagulant and stored at 4°C at ICAR-NAE laboratory at Veterinary college, Hebbal, Bengaluru for further processing. Blood samples were screened by Giemsa staining and PCR
Blood Smear Examination
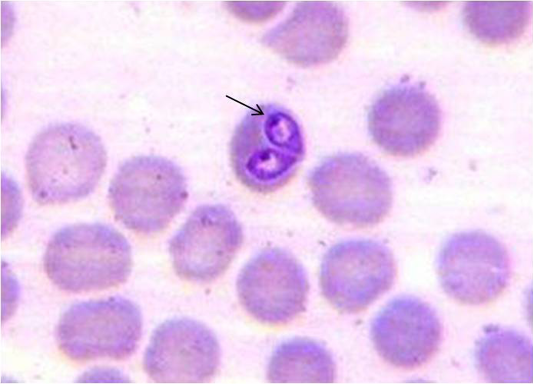
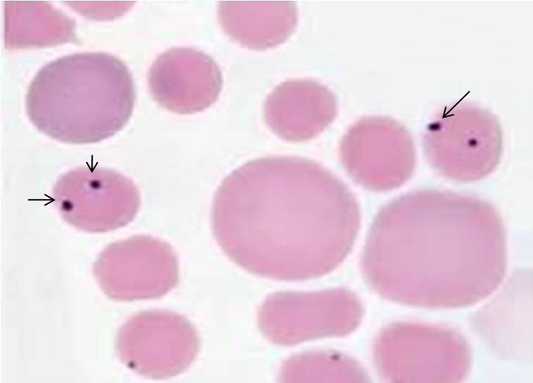
A drop of well mixed fresh blood sample was used to prepare blood smear and stained with Giemsa stain and examined under microscope for the presence of intra-erythrocytic babesia organisms. B.canis and B.gibsoni were differentiated based on their size (large or small) and appearance inside the erythrocytes (Soulsby, 1982).
Polymerase Chain Reaction
DNA was extracted from all the blood sample using QIA amp DNA blood mini kit (QIAGEN, GmbH, Germany) as per manufacturer’s instruction. DNA samples were amplified using B.canis and B.gibsoni species specific primers published by (Foldvari et al., 2005; Inokuma et al., 2004) respectively with slight modifications (Table 1). The specific amplicon size 450 bp (B. canis) and 671 bp (B. gibsoni) was analyzed by 1.0 % agarose ethidium bromide gel electrophoresis.
RESULTS
Of 102 samples screened,40 (39.21 %) and 66 (64.70 %) were positive by blood microscopy and PCR respectively (Table 2). Out of 40 blood microscopy positive samples 8 (7.84 %) and 32 (31.37 %) were positive for B. canisand B. gibsoni respectively (Figure 1 and 2). Out of 66 PCR positive samples, 23 (22.55 %) and 18 (17.65 %) were positive for B. canis and B. gibsoni respectively and 25 (24.50 %) samples were positive for both B. canis and B. gibsoni. (Figure 3 and 4)
Characteristic clinical features observed among 66 positive clinical cases were fever/pyrexia (77.5%), Anorexia (76%), history of ticks presence (73%), Lymphadenopathy (68%), Depression and lethargy (53%), Pale mucous membrane (45.5%), Loss of body weight (39.5%), Congested mucous membrane (38%), Respiratory distress (30%), Weakness (18%), Ocular discharge (18%), Nasal discharge (16.5%), Lameness (15%), Vomiting (13.5%), Diarrhea (7.5%), icteric mucous membrane (7.5%), Haematuria (6%) and Epistaxis (3%). In addition to the above signs splenomegaly (10.5%) and hepatomegaly (9%) were recorded based on abdominal ultrasonography. Neurological signs such as circling, trembling were recorded in 1.5% of cases.
In the present study out of 66 positive cases, higher incidence of canine babesiosis was seen in the age group of >1 – 2 years (23%) and lesser was in >8 - 10 years dogs (3%). The order of susceptibility observed was, > 1 – 2 years (23.0%), > 3 – 4 years (20%), > 2 – 3 years (15.0%), > 4 – 5 years (9.0%), > 6–12 months (7.5%), > 7 – 8 years (7.5%), 0 – 6 months (6.0%), > 6 – 7 years (3%), >8 – 9 years (3.0%), > 9 – 10 years (3.0%), > 5 – 6 years (1.5%) and > 10 – 11 years (1.5%). In Gender wise incidence, 38 (57.5%) were male dogs and 28 (42.5%) were female which indicated that male dogs had higher incidence than female.
The breed-wise incidence was higher in Labrador Retrievers (26%) compared to other breeds. The order of susceptibility was, German shepherd (17%), Cocker Spaniel (10.5%), Non-descriptive (9.0%), Golden Retriever (7.5%), Pug (7.5%), Rottweiler (4.5%), Dalmatian (3%), Great dane (3%), Siberian huskey (1.5%), Spitz (1.5%), Mudhol hound (1.5%), Irish setter (1.5%), Boxer (1.5%), Saint Bernard (1.5%), Pit bull terrier (1.5%) and Toy fox terrier (1.5%).
DISCUSSION
Giemsa stained blood smear examination findings in the present study corroborates with the results of (Laha et al., 2013; Das et al., 2015). However higher percentage of incidence has been reported by (Porchet et al., 2007; Senthil Kumar et al., 2009; Bhattacharjee et al., 2013). These variations in the occurrence of canine babesiosis could be attributed to prevalence of tick population, season, immune status of the host and other managemental practices.
In the present study PCR detected more number of samples as compared to blood smear examination indicating higher sensitivity and specificity of PCR which corroborates with the findings of (Macintire et al., 2002; Porchet et al., 2007; Laha et al., 2013).The clinical findings observed in the present study were similar to the observations made earlier by (Levine, 1973; Lobetti, 1998; Irwin, 2005; Loretti and Barros, 2005; Mathe et al, 2005).
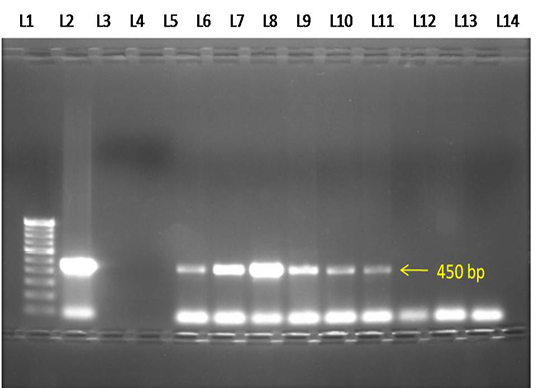
Figure 3: Electrophoresis gel (1.0 % agarose), showing lanes L1-100 bp DNA marker, L2- Positive control, L3- Negative control, L4- No template control, L5 to L10- Test samples showing positive PCR product of B.canis (450 bp), L11 to L13- Negative test samples.
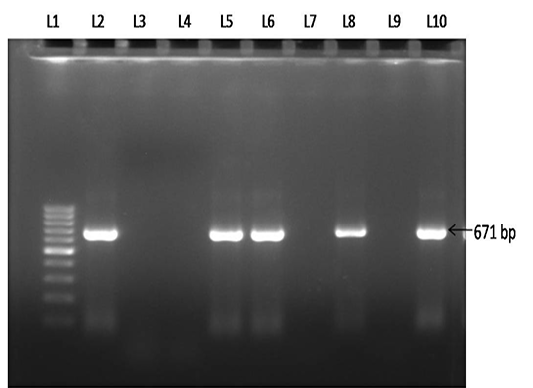
Figure 4: Electrophoresis gel (1.0 % agarose, stained with ethidiumbromide), showing lanes L1-100 bp DNA marker, L2- Positive control, L3- Negative control, L4- No template control,L5, L6, L8 & L10- Test samples showing positive PCR product of B.gibsoni (671 bp), L7 & L9- Negative test samples.
In the present study, higher incidence of canine babesiosis was found in the age group of >1– 2 years (23%) which correlates with the findings of (Singh et al., 2012; Amritpal Singh et al., 2014; Parveen kumar et al., 2015), which could be due to the underdeveloped immune system.
Table 1: Species specific primers used for the diagnosis of B.canis and B.gibsoni using PCR
|
Primer Name |
Primer Sequence |
Target Parasite |
Expected Ampliqon Size |
Reference |
|
PIRO-A1 PIRO-B |
F5’ AGGGAGCCTGAGAGACGGCTACC 3’ R5’ TTAAATACGAATGCCCCCAAC 3’ |
B.canis |
450 bp |
Foldvari et al. (2005) |
|
Gib599 Gib 1270 |
F5’ CTCGGCTACTTGCCTTGTC 3’R5’GCCGAAACTGAAATAACGGC 3’ |
B.gibsoni |
671 bp |
Inokuma et al. (2004) |
Table 2: Screening of dogs for Canine babesiosis by blood smear examination and PCR
|
Diagnostic tests |
Samplesscreened (n) |
Positives |
|
|
(n) |
(%) |
||
|
a. Microscopy ( Blood smear) |
102 |
40 |
39.21 |
|
b. PCR |
102 |
66 |
64.70 |
|
Total |
204 |
106 |
51.96 |
in young dogs as compared to adults. Many authors reported babesiosis in different age group of dogs. Therefore it can be opined that the age is not the criteria for babesia infection and depends on the transmitting vector and the immune status of the host.
In the present study male dogs (57.5%) were most commonly affected than females, which is in agreement with the findings of (Ilie et al., 2010; Nalubamba et al., 2015). However (Sandor Hornok et al., 2006; Senthil Kumar et al., 2009; Amuta et al., 2010; Amritpal Singh et al., 2014) have opined that breed of the host was not significantly associated with the incidence of babesiosis.
CONCLUSION
Overall prevalence of canine babesiosis was 51.9% both by blood smear examination and PCR. B. canis and B. gibsoni were detected in 30.3% and 49.0% dogs, respectively. Mixed infections with both B. canis and B. gibsoni were detected in 24.5% dogs. Prevalence of Canine babesiosis was ‘moderately high’ in dogs in and around Bengaluru. Higher prevalence may be attributed to the increased population of tick vectors and lower immune status of the host.Hence, proper control measures for ecto-parasite should be followed in dogs. Utility of multiple diagnostic tests is suggested for confirmatory detection and epidemiological diseases investigations of canine babesiosis in dogs.
Conflict of interest
We declare that we have no conflict of interest.
Acknowledgements
Authors are thankful to Indian Council of Agricultural Research (ICAR) and Karnataka Veterinary Animal and Fisheries Sciences University (KVAFSU), for provision of funding and facilities through ICAR -Niche Area of Excellence project ‘Animal Disease Registry and Tissue Bank’, Department of Veterinary Pathology, Veterinary College, Hebbal, Bengaluru.
AUTHOR’S CONTRIBUTION
All authors contributed equally.
REFERENCES