Advances in Animal and Veterinary Sciences
Case Report
Thalidomide Treatment in a Canine Mammary Gland Carcinosarcoma Presenting Pulmonary Metastasis
Cecilia Bonolo de Campos1,2; Gleidice Eunice Lavalle3; Silvia Fialho Ligório4; Lidianne Narducci Monteiro2; Renee Laufer Amorim5; Geovanni Dantas Cassali2
1Department of Veterinary Clinic and Surgery, School of Agricultural and Veterinary Sciences of the Sao Paulo State University (FCAV/UNESP) – Jaboticabal Campus, Brazil; 2Laboratory of Comparative Pathology, Department of General Pathology, Federal University of Minas Gerais (UFMG), Brazil; 3Department of Veterinary Clinic and Surgery, Veterinary School, Federal University of Minas Gerais (UFMG), Brazil; 4Department of Pharmaceutical and Biotechnological Development, Fundação Ezequiel Dias, Brazil;5Department of Veterinary Clinics, College of Veterinary Medicine and Animal Science, Sao Paulo State University (FMVZ/UNESP) – Botucatu Campus, Brazil.
Abstract | Carcinosarcomas are uncommon in the dog and present an unfavorable prognosis. Thalidomide has been used in the investigational treatment of several diseases due to its known immunomodulatory and anti-angiogenic properties. A female dog underwent radical unilateral mastectomy, which enabled the diagnosis of a stage III and grade III carcinosarcoma, followed by chemotherapy treatment with doxorubicin and carboplatin. Twelve months after the mastectomy, thoracic radiographs revealed the presence of multiple nodules in the lung, and thalidomide administration was initiated at 20 mg/kg/day during three months and then 10 mg/kg/day without discontinuation. The patient did not present any adverse events related to the thalidomide administration and thoracic radiographs demonstrated stable metastatic disease. The patient was euthanized presenting metastasis in several other organs and overall survival was considered 963 days. The progression of distant metastasis in the studied patient was considered hindered by thalidomide.
Keywords | Adjuvant therapy, Dog, Metastasis, Neoplasm, Survival.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | December 11, 2016; Accepted | January 26, 2017; Published | March 15, 2017
*Correspondence | Geovanni Dantas Cassali, Laboratory of Comparative Pathology, Department of General Pathology, Federal University of Minas Gerais (Ufmg), Brazil; Email: [email protected]
Citation | Campos CB, Lavalle GE, Ligório SF, Monteiro LN, Amorim RL, Cassali GD (2017). Thalidomide treatment in a canine mammary gland carcinosarcoma presenting pulmonary metastasis. Adv. Anim. Vet. Sci. 5(3): 120-126.
DOI | http://dx.doi.org/10.14737/journal.aavs/2017/5.3.120.126
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Campos et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Mammary gland neoplasms are the most common neoplasm in intact bitches (Brodey et al., 1983). The biological behavior of these tumors varies considerably, and prognosis is influenced by several factors, such as: age, histological type, clinical stage, tumor size, clinical behavior of the tumor, mitotic index, histologic grade, regional or distant metastasis, microvessel density, and molecular markers (Sorenmo, 2003; Cassali et al., 2014). All malignant mammary gland neoplasms have the potential to metastasize, however, histological type and several clinical prognostic factors influence the metastatic risk (Sorenmo, 2003). An increase in clinical stage worsens prognosis, and distant metastasis is associated to an accelerated demise of the affected animal (Yamagami et al., 1996).
Surgical excision of canine mammary gland neoplasms is considered the standard treatment modality. However, dogs with more undifferentiated and advanced tumors may require adjuvant therapy (Novosad, 2003; Sorenmo, 2003). Chemotherapy should be recommended when the histotype of the primary neoplasm is associated with poor prognosis, i.e., solid carcinomas, micropapillary carcinomas, anaplastic carcinomas and carcinosarcomas, or when patients present metastasis (Cassali et al., 2014; Nunes, 2015). Chemotherapy may also be used in the adjuvant setting and in dogs with gross metastatic disease (Sorenmo, 2003).
Despite thalidomide’s tragic history, thalidomide has been used in the investigational treatment of a myriad of diseases (Paravar and Lee, 2008). In humans, therapeutic activity of the drug has been described for non-neoplastic diseases, e.g. erythema nodosum leprosum, and neoplastic diseases, e.g. multiple myeloma (Singhal and Mehta, 2002; Dimopoulos and Eleutherakis-Papaiakovou, 2004). The drug is usually given as a single daily dose in the evening, due to somnolence and orthostatic hypotension, and teratogenicity is the most serious adverse effect (Adlard, 2000). The known immunomodulatory and anti-angiogenic properties of thalidomide and its analogues provided the impetus to investigate these agents in the treatment of both hematologic malignancies and in solid tumors. Ongoing trials, either in combination with chemotherapy or as single agents, have been initiated in neoplastic and non-neoplastic diseases (Melchert and List, 2007).
Currently, there are insufficient clinical trials concerning the use of thalidomide in veterinary medicine. Therefore, the aim of this paper is to report a case of thalidomide induced inhibition of progression of distant metastasis of a canine mammary gland carcinosarcoma.
An eleven-year-old schnauzer presenting mammary gland tumors was admitted at the Veterinary Hospital of the Federal University of Minas Gerais, Brazil. Thoracic radiographs (TR), including right and left lateral recumbent (RLR and LLR) and ventrodorsal (VD) projections and abdominal ultrasound (AU) did not demonstrate distant metastasis. The patient was classified as clinical stage III (T3N0M0) (Sorenmo et al., 2013) and underwent radical unilateral mastectomy.
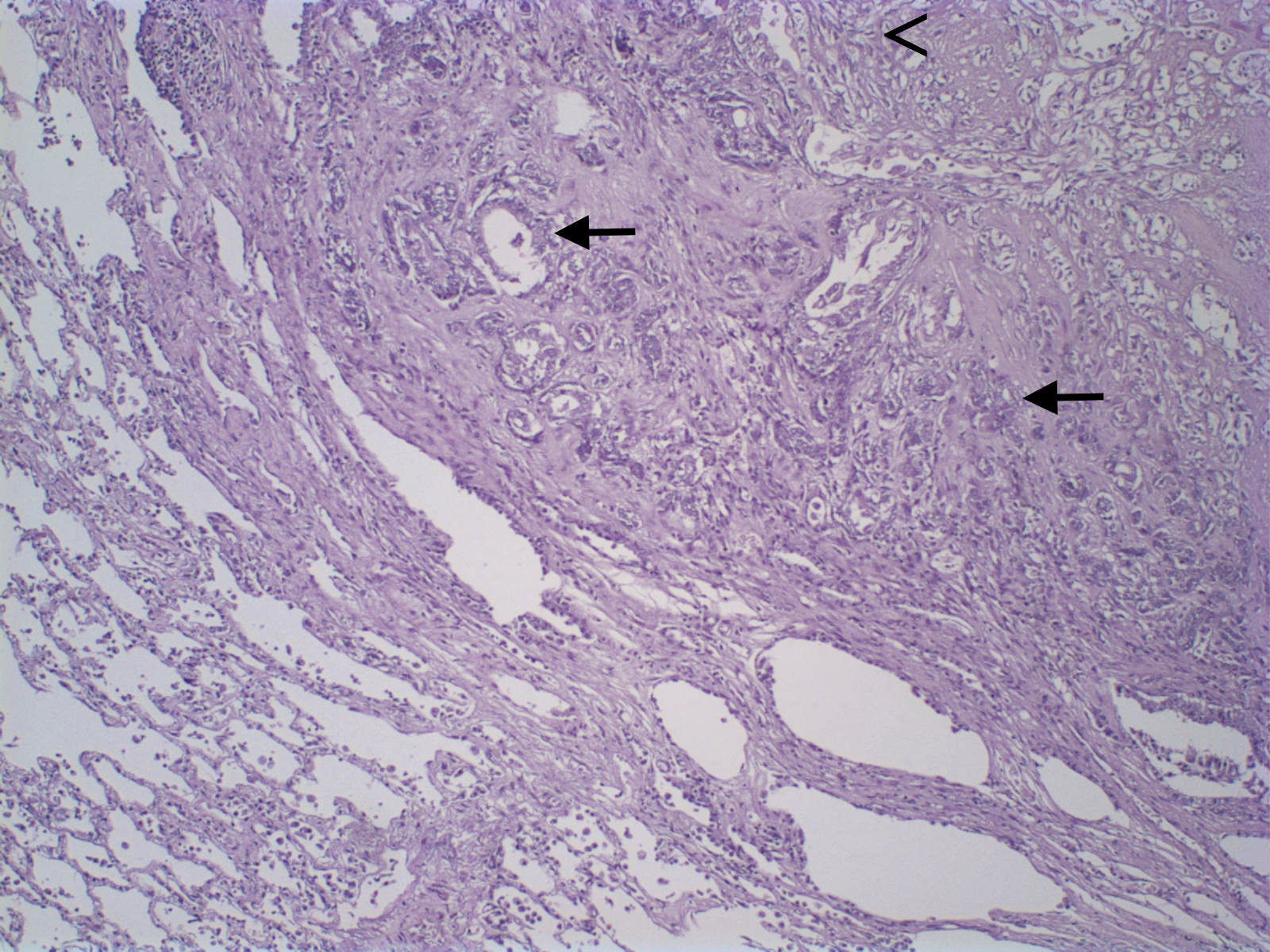
Mammary gland tumors were fixed in 10% neutral buffered formalin and embedded in paraffin and 4-µm thick histologic sections were stained with hematoxylin and eosin and classified according to WHO’s Histological Classification for canine and feline mammary tumors (Misdorp et al., 1999). Macroscopic evaluation of the primary neoplasm demonstrated a 5.0 x 3.0 x 1.5 cm nodule in the right cranial abdominal mammary gland. Microscopic analysis revealed epithelial cells arranged in a predominantly tubular pattern associated with spindle-shaped cells. Epithelial cells were characterized by prominent nucleoli and moderate cellular pleomorfism, with an average of two mitoses per high power field (400x). Spindle cells presented scattered mitotic figures, moderate cellular pleomorfism, marked anisocytosis and anisokaryosis, nuclear hypercromasia, and occasional multinucleated cells. Based on histologic findings the neoplasm was diagnosed as a carcinosarcoma (Figure 1). Histological grade was established according to the Nottingham system (Elston and Ellis, 1998), and the primary neoplasm was considered grade III. In addition, the patient presented a 0.4 cm nodule in the cranial thoracic mammary gland, diagnosed as a carcinoma in mixed tumor, and the inguinal lymph node was negative for metastasis.
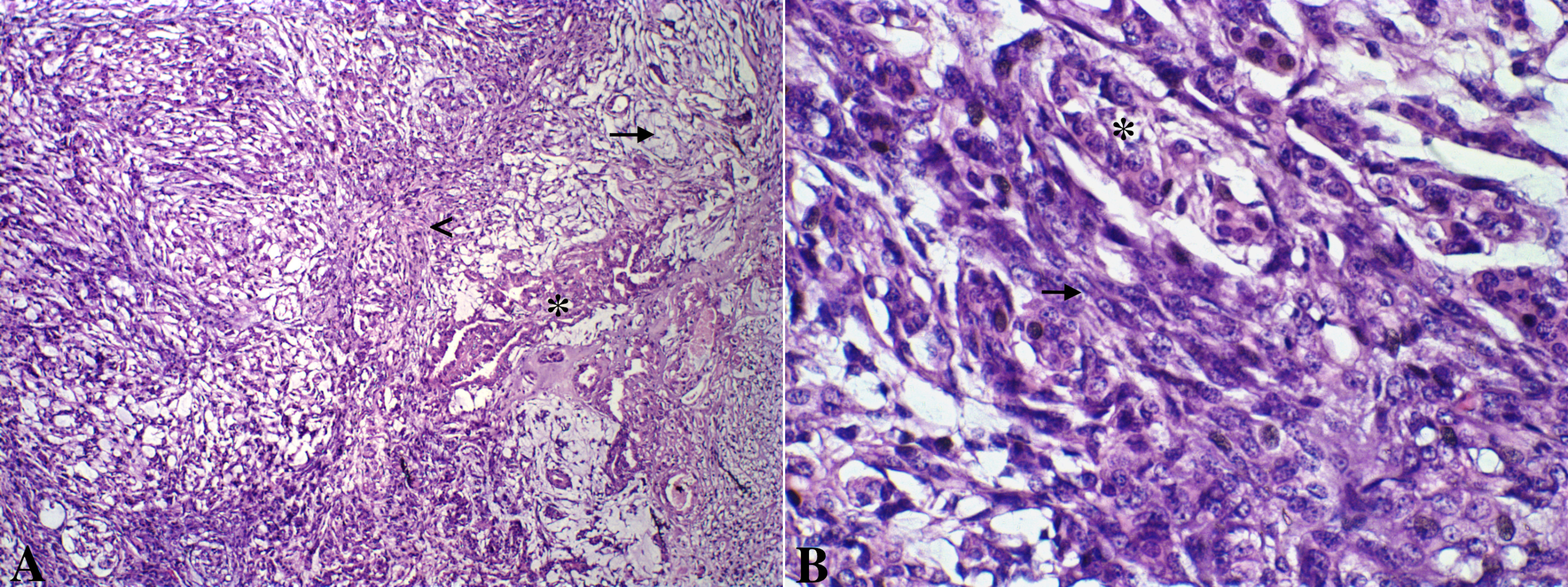
Figure 1: A. Canine mammary gland carcinosarcoma presenting a neoplastic epithelial component (asterisk) and a mesenchymal component with spindle cells(arrow head) and myxoid matrix (arrow). Hematoxylin and eosin, x100. B. Greater details of epithelial (asterisk) and mesenchymal spindle cells (arrow) shown. Hematoxylin and eosin, x600.
Due to the histological diagnosis, surgical excision was followed by chemotherapy, consisted by four cycles of an alternating combination of doxorubicin at 30 mg/m2 and carboplatin at 300 mg/m2, given intravenously every 21 days, and follow-up was subsequently performed every three months with clinical evaluation, TR, and AU of the patient.
Twelve months after the mastectomy, TR revealed the presence of multiple nodules in the lung, not associated with respiratory clinical signs. Thalidomide administration was initiated at 20 mg/kg, given orally every 24 hours at night time, during three months. Afterwards, thalidomide was given orally at 10 mg/kg every 24 hours at night time, without discontinuation. The patient did not present any adverse events related to the thalidomide administration.
TR analysis demonstrated a slow progression of the metastatic lung disease, classified as a stable disease (increase lower than 25% in tumor maximum diameter) (Figure 2). After fifteen months of the thalidomide treatment, an AU demonstrated the presence of a hyperechogenic splenic nodule of 1.65 x 1.66 cm and the animal was submitted to a splenectomy. Histopathological analysis revealed a distant metastastatic lesion of the primary carcinosarcoma in the spleen.
Twenty months after the diagnosis of the pulmonary nodules and the beginning of the thalidomide treatment, the patient was submitted to euthanasia procedures as a result of a progressive peripheral vestibular syndrome. Overall survival was considered 32 months (963 days), defined as the period between the date of surgical removal of the tumor and death caused by the disease.
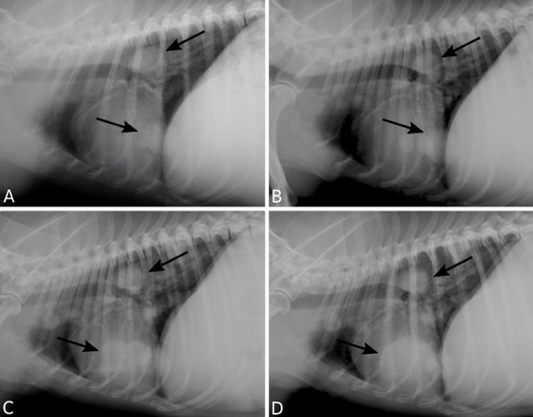
Figure 2: Pulmonary metastasis of a canine mammary gland carcinosarcoma. Right lateral recumbency thoracic radiographs (TR) presenting multiple nodules (arrows). TR performed 12 (A), 18 (B), 24 (C), 30 (D) months following surgical excision of the primary neoplasm.
In addition, the dog was submitted to post-mortem evaluation and gross metastasis were revealed in the lung, mediastinal and mesenteric lymph nodes, liver, pancreas, and adrenal glands, as well as neoplastic emboli within central nervous system. Metastasis were subsequently confirmed through microscopic analysis, with cells presenting similar histologic features to those observed in the primary tumor (Figure 3).
Morphometric analysis of the inflammatory infiltrate was performed according to (Estrela-Lima et al., 2010). Inflammation within the primary neoplasm and the pulmonary distant metastasis were considered multifocal and intense, with similar quantification of inflammatory cells, presenting an intense lymphocytic component (Figure 4).
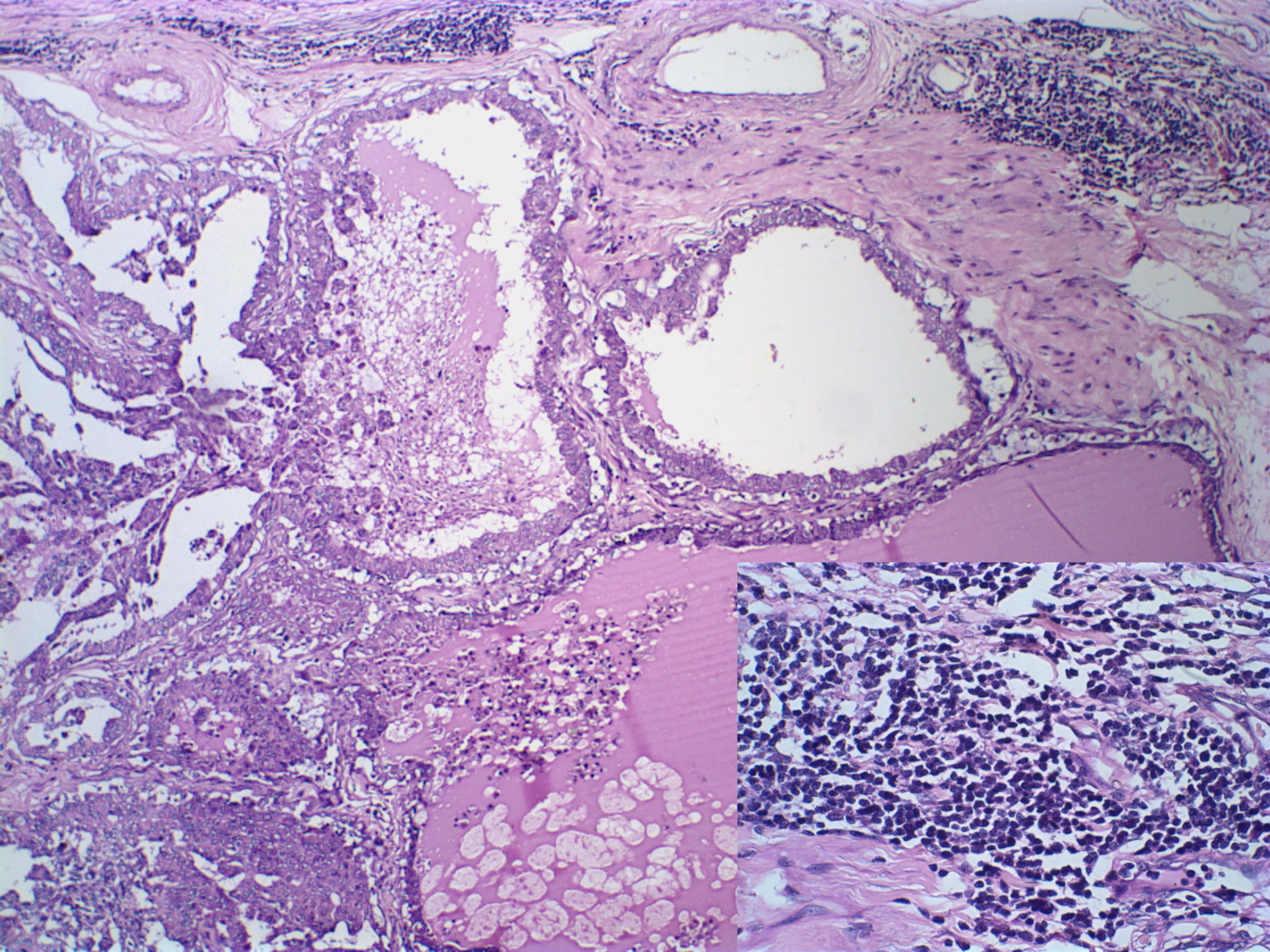
Figure 4: Canine mammary gland carcinosarcoma associated with a multifocal and intense inflammatory infiltrate, presenting an intense lymphocytic component (insert, Hematoxylin and eosin, x1000). Hematoxylin and eosin, x600.
Three µm-thick histologic sections from the primary neoplasm and lung metastasis were obtained for immunohistochemistry. Slides were incubated following the details provided in Table 1. A polymeric-based detection system (Novolink Polymer Detection System, Novocastra, Newcastle, UK) was used for detection of antigen-antibody reaction and 3,3’-Diaminobenzidine was used as chromogen. Slides were subsequently counterstained using Harris hematoxylin. Sections from a canine mammary carcinoma known to express Ki-67, ER and PR, COX-2, and CD31 were used as positive controls. Negative controls were assessed using normal serum (Ultra V Block, Laboratory Vision) as the primary antibody.
The primary neoplasm presented a proliferation index of 18.1%, was positive for estrogen and progesterone receptors, Cox-2 score 2, and a microvessel density of 33/200x field. Lung metastasis presented a proliferation index of 9.4% and a microvessel density of 61/200x field (Figure 5).
The lungs are the most common site for distant metastasis in dogs with malignant mammary gland tumors (Sorenmo, 2003). The absence of salient clinical abnormalities associated with pulmonary metastatic disease leads to considerable attention on thoracic radiography as a diagnostic, prognostic, and staging tool (Miles et al., 1990). However, additional staging tests, including abdominal ultrasonography and abdominal and skeletal radiographs, may be indicated (Sorenmo, 2003; Sorenmo et al., 2011).
Table 1. Target antigen and clone, dilution, antigen retrieval method, and incubation time and temperature for immunohistochemical staining for Ki-67, Estrogen Receptor (ER), Progesterone Receptor (PR), Cyclooxigenase-2 (Cox-2), and CD31.
|
Antigen |
Clone |
Dilution |
Antigen Retrieval Method / Buffer |
Incubation Time (hd) / Temperature |
IHC Evaluation |
|
Ki-67 |
MIB-1 |
1:50 |
PHa (125°C/2min) / Citrate |
16 / 4oC |
(Dutra and others 2008) |
|
ER |
1D5 |
1:20 |
PH (125°C/2min) / EDTA |
1 / RTc |
(Hammond and others 2010) |
|
PR |
HPRA2 |
1:20 |
PH (125°C/2min) / EDTA |
1 / RT |
(Hammond and others 2010) |
|
Cox-2 |
SP21 |
1:80 |
WBb (98°C/20min) / Citrate |
1 / RT |
(Lavalle and others 2012) |
|
CD31 |
JC70A |
1:100 |
PH (125°C/2min) / Citrate |
16 / 4oC |
(Weidner 1995) |
aPressurized Heat bWater Bath cHours dRoom Temperature
Three view TR are necessary for all dogs with malignant mammary gland neoplasms, which remains as the standard diagnostic method for the evaluation of thoracic metastatic disease in veterinary medicine. Conventional radiography can detect lesions ranging from 6-9 mm in diameter (Sorenmo, 2003; Nemanic et al., 2006). Radiographic detection of lung masses depends on several intrinsic (location, size, mass opacity, normal thoracic and extrathoraric structures) and radiographic technique factors, as well as the interpretation of radiographs. The RLR projection is the most sensitive for detection of lung metastasis, followed by the LLR and the VD projection. The sensitivity for the three-view combination is nearly 100%, regardless of the number of readers (Lang et al., 1986).
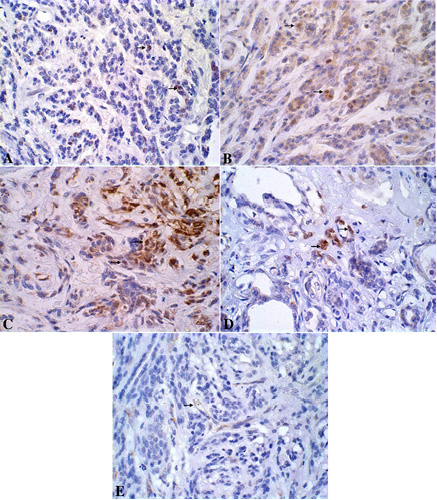
Figure 5: A. Canine mammary gland presenting neoplastic Ki-67-immunoreactive epithelial cells stained in brown (nucleous). DAB immunohistochemistry, counterstained with Harris’s hematoxylin, x600. B. Canine mammary gland presenting neoplastic ER-immunoreactive epithelial cells stained in brown (nucleous). DAB immunohistochemistry, counterstained with Harris’s hematoxylin, x600. C. Canine mammary gland presenting neoplastic PR-immunoreactive epithelial cells stained in brown (nucleous).
There are no established guidelines for the treatment of malignant mammary gland neoplasms beyond surgery. However, treatment recommendations generally intensify with advancing clinical stage and increasing seriousness of the prognostic factors (Sorenmo, 2003). Carcinosarcomas present extremely variable histological characteristics (Misdorp et al., 1973) and are uncommon in the dog (Misdorpet al., 1999) presenting very aggressive biological behavior and an unfavorable prognosis (Von Euler, 2011). The lungs are the most frequent site of metastasis, followed by regional lymph nodes. Carcinosarcoma metastasis are characterized by carcinomatous, sarcomatous, or mixed components. Other sites less frequently affected are heart, kidneys, liver, adrenal glands, brain, ovaries, hypophysis, bones, spleen, and pleura. The average time between detection and death in seven dogs was 18 months (Misdorp et al., 1973).
Thalidomide was described as a potent angiogenesis inhibitor in vivo (D’Amato et al., 1994). The discovery of thalidomide’s antiangiogenic properties coincided with the emerging importance of angiogenesis in tumor growth and progression (Richardson et al., 2002). The anti-angiogenic activity of thalidomide suggest that the drug may present potential clinical benefit in the treatment of malignancies in which neoangiogenesis is present, including solid tumors (D’Amato et al., 1994; Calabrese and Fleischer, 2000; Melchert and List, 2007). Antiangiogenic therapy is an appealing strategy for targeting resistant disease, and accumulating evidence suggests that the combination of cytotoxic chemotherapy and antiangiogenic therapy has greater antitumor effects than either strategy alone (Richardson et al., 2002). Therefore, a novel treatment protocol option for advanced canine mammary gland tumors may consist of surgery, chemotherapy and thalidomide in association, although clinical trials are necessary to verify the clinical benefit of the proposed therapeutic protocol.
Thalidomide and its analogs exhibit a multitude of biologic effects on cytokine and cell-mediated responses (Melchert and List, 2007). The effects of thalidomide on immune function are incompletely understood; however, anti-inflammatory and immunomodulatory activities have been described (Calabrese and Fleischer, 2000).
The antiangiogenic and immunomodulatory properties of thalidomide are implicated in the major activity of the drug in patients with multiple myeloma and holds promise in the treatment of other hematologic malignancies. Results in solid tumors are less encouraging, but patients with certain tumors, such as glioma, renal cell cancer, and prostate cancer, may benefit (Richardson et al., 2002). Our results demonstrated an increase in the microvessel density at the metastatic site when compared to the primary neoplasm. The inflammatory infiltrate was similar in the primary and metastatic neoplasms. Therefore, the antiangiogenic and immunomodulatory properties of the drug were not demonstrated. However, the proliferative index of the primary neoplasm was higher than the metastatic proliferative index, which may suggest the antitumor effect of the drug. Direct cytotoxic effect of thalidomide on tumor cells was demonstrated on multiple myeloma cell lines, with inhibition of DNA synthesis in vitro (Hideshima et al., 2000).
In canines, the safety profile of thalidomide was evaluated and daily oral administration was considered well tolerated. The NOAEL (no-observed-adverse-effect levels) was 200 mg/kg (Teo et al., 2001). Two studies began to evaluate the clinical benefit of thalidomide in canine malignancies; however, the clinical trials were not completed (Jankowski et al., 1999; Woods et al., 2004). The present case report suggests that the drug provided clinical benefit in the treatment of distant metastasis. In addition, the patient maintained adequate quality of life during the thalidomide treatment, with an absence of adverse effects.
Gross metastatic disease at the time of diagnosis was associated to a poorer prognosis, with a median post-operative survival of 5 months, when compared to 28 months for animals diagnosed with mammary gland neoplasms that lacked evidence of metastasis at diagnosis (Philibert et al., 2003). Another study found a 13.6% survival rates one year after mastectomy for animals diagnosed with distant metastasis (Yamagami et al., 1996). In veterinary oncology, there are insufficient options for the treatment of distant metastasis. However, the 20-month overall survival after the diagnosis of pulmonary nodules in the present case report was considered satisfying.
The progression of distant metastasis in the studied patient was considered hindered by thalidomide. However, clinical trials are necessary to confirm the benefit of thalidomide in canine mammary gland neoplasms, as well as in other malignant neoplasms in veterinary medicine.
Acknowledgements
This research was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP Grant 2014/01329-9) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Grant 302449/2013-2).
Conflict of Interest
The authors declare they have no conflict of interest.
Authors Contribution
CBC, GEL, and GDC conceived the study. CBC performed data collection and wrote the manuscript. CBC and LDM performed data analysis. SLF aided in the obtainment of necessary resources. GDC, GEL, RLA, SLF supervised the work. All authors were involved in revising the manuscript.
References