Advances in Animal and Veterinary Sciences
Research Article
Prevalence of Ovine Johne’s Disease in Bannur Breed of Sheep in Organized Farm using Multiple Diagnostic Tests
Shivalingappa Yamanappa Mukartal1, Doddamane Rathnamma1, Hogalagere Doddappaiah Narayanaswamy2, Shrikrishna Isloor1, Shoorvir Singh3*, Basavegowdanadoddi Marinaik Chandranaik4, Shobha Rani Methuku4, Anuradha Menon Elattuvalappil2, Srikanth Mallaiah2, Manjunath Suranagi Shambanna5
1Department of Veterinary Microbiology; 2Department of Pathology, Veterinary College, Hebbal, Karnataka Veterinary Animal and Fisheries Sciences University (KVAFSU), Bengaluru-560024; 3Division of Animal Health, Central Institute for Research on Goats (CIRG), Makhdoom, PO-Farah, Mathura-281122, Uttar Pradesh, India; 4Institute of Animal Health and Veterinary Biologicals (IAH&VB), Hebbal , Bengaluru-560024; 5Livestock Research and Information Centre (Sheep) Karnataka Veterinary Animal and Fisheries Sciences University (KVAFSU), Nagamangala-571432, India.
Abstract | Ovine Johne’s disease (OJD) is a chronic incurable inflammation of intestines caused by Mycobacterium avium subspecies paratuberculosis (MAP). Prevalence of OJD was estimated in the Bannur breed of sheep located in Mandya district of Karnataka state on the basis of clinical signs and detecting presence of bacilli in faeces and antibodies in serum of naturally infected sheep. Clinical samples (faeces-135, blood-45 and serum-100) were collected from sheep located at LRIC (Livestock Research and Information Centre) farm and Dangur sheep breeding farm. Sheep at LRIC farm (>15.0%) exhibited clinical symptoms of sub-mandibular oedema, emaciation, alopecia and were suspected for JD. None of the sheep at Dangur farm, had clinical symptoms of JD. Of 168 samples (LRIC farm), 30 (60.0%), 38 (76.0%), and 24 (35.2%) were positive by microscopy, ELISA and IS900 PCR, respectively. Whereas of 112 samples (Dangur farm), 5 (10.0%), 7 (14.0%) and 6 (50.0%) were positive by microscopy, ELISA and IS900 PCR respectively. Overall prevalence of JD was 54.7 and 16.0% in LRIC and Dangur farms, respectively. Microscopy and ELISA were screening tests and PCR was confirmatory. Prevalence of OJD was moderately high in Bannur breed of sheep and combinations of microscopy and ELISA followed by PCR may be adopted as a strategy for screening and diagnosis of JD in sheep.
Keywords | Johne’s disease, Mycobacterium avium subspecies paratuberculosis, Microscopy, ELISA, IS900 PCR
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | September 21, 2016; Accepted | September 28, 2016; Published | October 07, 2016
*Correspondence | Shoorvir Singh, Division of Animal Health, Central Institute for Research on Goats (CIRG), Makhdoom, PO-Farah, Mathura-281122, Uttar Pradesh, India; Email: [email protected]
Citation | Mukartal SY, Rathnamma D, Narayanaswamy HD, Isloor S, Singh S, Chandranaik BM, Methuku SR, Elattuvalappil AM, Mallaiah S, Shambanna MS (2016). Prevalence of ovine Johne’s disease in bannur breed of sheep in organized farm using multiple diagnostic tests. Adv. Anim. Vet. Sci. 4(10): 506-512.
DOI | Http://dx.doi.org/10.14737/journal.aavs/2016/4.10.506.512
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Mukartal et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Johne’s disease (JD) is an economically important chronic and fatal disease affecting domestic and wild ruminants and is caused by Mycobacterium avium subspecies paratuberculosis (MAP). MAP strains have been bio-typed as ‘Cattle type’, ‘Sheep type’ and Intermediate or Indian Bison type’ (Whittington et al., 2001; Sevilla et al., 2005). All three sub-types can cause disease in all ruminant species (Quinn et al., 2011). In small ruminants, clinical signs characteristics to JD are not as obvious as in cattle (Juste and Perez, 2011). In small ruminants, diarrhoea is not an important clinical feature instead the characteristic observation was chronic weakness, weight loss and emaciation which is also seen in many other diseases. Only 10–20% clinically infected small ruminants may develop diarrhoea at the final stages of disease leading to the spread of disease in flocks that goes unnoticed (Smith, 2004). Economic losses are mainly due to lowered slaughter value, early culling and finally death (Ott et al., 1999). Several reports suggest that MAP is associated with inflammatory bowel disease or Crohn’s disease in human beings which is of public health importance (Chamberlin et al., 2001).
Only few recent studies in India reported wide variations in prevalence (15.1-36.0%), of OJD (Singh et al., 2009; Gupta et al., 2012; Vinodhkumar et al., 2013). Country lacks rapid, sensitive and indigenous kits for the diagnosis of OJD. Long subclinical phase of disease leads to numerous undiagnosed cases of OJD, are major hurdles in the control of disease at farm level (McKenna et al., 2006).
Microscopy, ELISA and PCR have been frequently used for the detection of MAP infection in animals (Singh et al., 2013). ‘Bannur’, is also known as ‘Mandya breed’ of sheep, distributed in Mandya district and bordering areas of Mysore districts of Karnataka. Information does not exist on the prevalence of JD in Bannur breed of sheep in Karnataka. Therefore, present study was undertaken to estimate prevalence of JD in Bannur sheep using multiple tests and evaluation of sensitivity and specificity of tests used for the diagnosis of JD in two sheep flocks.
Materials and Methods
Animals
Two farms (Livestock Research and Information Centre (LRIC), and Dangur sheep breeding farm (DSBF) located in Mandya district of Karnataka) of Bannur breed of sheep suspected with clinically Johne’s disease (Figure 1) (poor in body conditions with low body weight, emaciation, sub-mandibular oedema, rough hair coat and loss of hairs) were sampled.
Collection of Samples and Tests
Fifty sheep from each farm were sampled (faeces, serum and blood). A total of 280 samples (serum =100; faeces = 135; blood = 45) were collected. Samples were collected in sterilized containers and processed at ICAR-NAE laboratory at Veterinary college, Hebbal, Bengaluru and stored at -20˚C for further use. Faeces and serum samples were screened by acid fast staining and “Indigenous ELISA” tests, respectively (Figure 2). IS900 specific PCR was employed for screening of faecal and blood samples. Combinations of tests such as microscopy and ELISA, microscopy and faecal PCR and ELISA and blood PCR, were evaluated to know the best combination of tests for the diagnosis of MAP infection in sheep.
Faecal Microscopy
About 2-3 grams of fecal sample was collected in polythene bag from clinically suspected sheep, directly from rectum. Samples were homogenized and concentrated by centrifugation at 4500 rpm for 45 min at room temperature. Supernatant was discarded and from middle layer smears were prepared, stained by Ziehl Neelsen (ZN) staining and were examined under oil immersion (100X) for presence of acid-fast bacilli (AFB).
Indigenous ELISA
Serum samples collected during the study were screened by using ‘Indigenous ELISA kit’, developed by CIRG Makhdoom. Indigenous ELISA kit uses semi-purified protoplasmic antigen (PPA) from the highly virulent native isolate of MAP ‘Indian Bison type’ biotype of sheep origin (Singh et al., 2015a). OD values were transformed to S/P ratio and animals in positive and strong positive categories in S/P ratio were considered as positive for MAP infection.
DNA isolation and IS900 PCR
A total of 30 faecal samples and 38 blood samples were processed for DNA isolation as per Van Embden et al. (1993) and Singh et al. (2010b). Samples were screened for the presence of MAP in faecal and blood samples using IS900 PCR to obtain the frequency of distribution of MAP in Bannur sheep. DNA samples were amplified using specific IS900 (P90 and P91) primers published by Millar et al. (1996). Briefly, in a volume of 12.5 μl of 2X master mix, 0.5 μl forward primer (10 pmole/μl) and ) 0.5μl reverse primer (10 pmole/μl), 9.5 μl of nuclease free water and 2 μl of template DNA was added (total volume 25 μl). Total of thirty seven cycles were performed in a thermal cycler for complete amplification reaction. Thermal cycling conditions were initial denaturation at 94 °C for 5 min (1 cycle), followed by 37 cycles of denaturation at 94 °C for 30 s, annealing at 64 °C for 30 s, extension at 72 °C for 30 s and final extension at 72 °C for 7 min. The specific amplicon of 413bp product was analyzed by 1.5% agarose ethidium bromide gel electrophoresis (Figure 3).
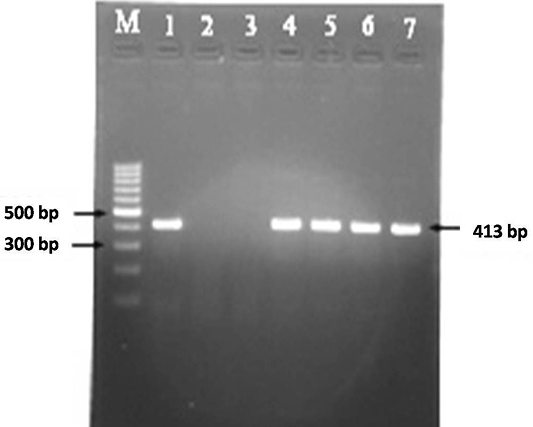
Figure 3: MAP specific amplicons (413 bp) by PCR using IS900 specific primers. Lane M: 100 bp ladder; Lane 1: Positive control (MAP DNA); Lane 2 and 3: Negative control and no template control; Lane 4-7: DNA samples isolated from faeces and blood
Statistical Analysis
Sensitivity, specificity and kappa agreement values between test combinations (Microscopy- ELISA, Microscopy- faecal PCR and ELISA- blood PCR) were analysed by using Graph Pad Prism version 5 software USA.
Table 1: Screening of Bannur breed of sheep for Johne’s disease using multiple diagnostic tests
|
Diagnostic tests |
Samples screened (n) |
Positives |
|
|
(n) |
(%) |
||
|
LRIC Farm Nagamangala |
|||
|
Microscopy |
50 |
30 |
60.0 |
|
Indigenous ELISA kit |
50 |
38 |
76.0 |
|
IS900 PCR |
68 |
24 |
35.29 |
|
Sub total |
168 |
92 |
54.76 |
|
Sheep breeding farm Dangur |
|||
|
Microscopy |
50 |
5 |
10.0 |
|
Indigenous ELISA kit |
50 |
7 |
14.0 |
|
IS900 PCR |
12 |
6 |
50.0 |
|
Sub total |
112 |
18 |
16.07 |
Results
Of 168 samples screened by multiple tests from LRIC farm 30 (60.0%), 38 (76.0%) and 24 (35.29%) were positive by microscopy, ELISA and IS900 PCR, respectively. Of 24 IS900 PCR positive samples 18 (60%) and 6 (15.7%) were positive in faecal and blood PCR, respectively (Table 1). Combination of tests detected 48 (60.0%), 68 (68.0%), and 44 (57.8%) of samples positive by Microscopy- faecal PCR, Microscopy- ELISA and ELISA- blood PCR, respectively. Value of kappa coefficient was good (0.720), between Microscopy- faecal PCR indicating good agreement. Between Microscopy-ELISA, moderate (0.561) agreement was observed whereas highest kappa value (0.774) with good agreement was observed in ELISA– blood PCR (Table 2).
Table 2: Comparative diagnostic potential of different test combinations for diagnosis of Johne’s disease infection
|
Test combinations |
Samples (n) |
Positive (%) |
Kappa value |
Strength of agreement |
Specificity (95% CI) |
Sensitivity (95% CI) |
|
|
LRIC Farm, Nagamangala |
|||||||
|
Microscopy + ELISA |
100 |
68 |
0.561 |
Moderate |
0.6667 |
0.8947 |
|
|
Microscopy + Faecal PCR |
80 |
48 |
0.720 |
Good |
0.8148 |
0.9057 |
|
|
ELISA + Blood PCR |
76 |
44 |
0.774 |
Good |
0.8571 |
0.9167 |
|
|
Sheep breeding farm, Dangur |
|||||||
|
Microscopy + ELISA |
100 |
12 |
0.593 |
Moderate |
0.9268 |
0.6667 |
|
|
Microscopy + Faecal PCR |
55 |
8 |
0.659 |
Good |
0.9318 |
0.7273 |
|
|
ELISA+ Blood PCR |
57 |
11 |
0.716 |
Good |
0.9302 |
0.7857 |
|
<0.20: poor; 0.21-0.40: fair; 0.41-0.60: moderate; 0.61-0.80: substantial (good); and 0.81-1.00: almost perfect
On comparison, sensitivity of ELISA- blood PCR was highest (0.9167), as compared to microscopy- faecal PCR (0.9057) and microscopy-ELISA. (0.8947). Specificity of ELISA- blood PCR was highest (0.8571) as compared to Microscopy- faecal PCR (0.8148) and Microscopy-ELISA (0.667) (Table 2).
Of 112 samples screened by multiple tests from Dangur sheep breeding farm 5 (10.0%), 7 (14.0%) and 6 (50.0%) were positive by microscopy, ELISA and IS900 PCR respectively (Table 1). Of six IS900 PCR positive samples, 3 (60%) and 3 (42.8%) were positive in faecal and blood PCR, respectively. Multiple diagnostic tests detected 8 (14.5%), 12 (12.0%), and 11(19.2%) samples positive by Microscopy- faecal PCR, Microscopy-ELISA and ELISA- blood PCR, respectively (Table 2).
Value of kappa coefficient was good (0.659), between microscopy- faecal PCR indicating good agreement. It was moderate (0.593) agreement between Microscopy- ELISA whereas highest kappa value (0.716) with good agreement was observed in ELISA- blood PCR (Table 2). On comparison, the sensitivity of ELISA- blood PCR was highest (0.7857) as compared to Microscopy- faecal PCR (0.7273), and Microscopy - ELISA (0.6667) (Table 2). Specificity was highest between microscopy- faecal PCR (0.9318), as compared to ELISA- blood PCR (0.9302) and microscopy-ELISA. (0.9268).
Discussion
Johne’s disease causes serious economic losses to sheep farmers. Effective control of Johne’s disease is hampered due to the lack of rapid and accurate diagnostic tests. Diagnosis and control of disease in sub-clinically infected animals is extremely challenging, since disease gets transmitted prior to the development of clinical signs (Sohal et al., 2007). Designing any ‘strategy’ for ‘disease control’ in sheep flocks it is essential to know the frequency and distribution of MAP infection. Many researchers identified and quantified management factors that may be associated with the disease to assess the prevalence of disease.
In the multiple stages of MAP infections, variable clinical signs were observed in different ruminant species, diarrhoea being the prominent clinical sign of the disease in cattle whereas it may not be present in majority of cases in sheep (Gilmour and Nyange, 1989; Chandranaik et al., 2014). In the present study >15.0% sheep showed the clinical signs resembling that of JD in LRIC farm, which included loss of appetite, dullness, emaciation, submandibular oedema, rough hair coat, alopecia, weight loss and no diarrhoea. In contrast, sheep of Dangur farm did not show clinical signs similar to JD, suggesting the need for accurate, field based indigenous tests for the diagnosis of JD in sheep. MAP infection in animals is traditionally diagnosed by microscopy, indirect ELISA test and IS900 PCR, however sensitivity and specificity of these tests varies with the stages of disease.
Current study detected higher percent of positive sheep in microscopy (60.0%) as compared to IS900PCR (35.2%) in LRIC farm. Sensitivity of microscopy depended largely on faecal shedding of MAP bacilli and specificity depend on experience of the user. These findings are in agreement with those of Singh et al. (2013), who reported 33.4% per cent in Bharat Merino sheep, located at Mannavanur of Tamil Nadu and Bikaner of Rajasthan regions. Reason for high prevalence of the MAP shedders in LRIC, farm might be due to the fact that disease was active in the farm. Samples were collected from sheep suffering from clinical symptoms of JD. In LRIC farm sheep flock was maintained completely on the grazing. Animals were housed in the common enclosure, therefore infected animals acted as source of infection to other sheep. In contrast, sheep at Dangur farm, faecal microscopy detected less number of positive animals compared to IS900 PCR Reasons for low incidence of MAP shedders might be due to sub-clinical stage of disease in the farm. These findings are in agreement with Chaudhry et al. (2012) who reported 17.4% prevalence in Lahore district of Pakistan. Manning et al. (2003) opined that even though sensitivity and specificity of microscopy was low, it helped in estimating rate of shedding of MAP in faeces of infected animals. Serological surveys are the best methods for assessing the prevalence of disease in endemic areas. Although specificity of tests is less as compared to culture but it is a cost effective method. As per the OIE (2000), ELISA is accredited to be the most sensitive and specific tool for detection of MAP infection and is the most effective flock screening test.
In the present study, ELISA detected more number of positive animals as compared to faecal microscopy and IS900 PCR in LRIC farm. Higher sero-prevalence of 92.9 and 30.7% has also been reported in Mathura and Mannavanur regions, respectively by Singh et al. (2014). Probable reason for the higher sero-prevalence in the present study might be due to increased incidence of the clinical disease in the flock and sheep were suffering from chronic infections which resulted in sero-conversion in the affected sheep. In contrast, sheep of Dangur farm ELISA detected less number of positive animals compared to IS900 PCR which might be due to sub-clinical form of disease. Similar, lower prevalence of 7.7% was reported in Bikaner of Rajasthan regions by Singh et al. (2014).
IS900 PCR proved to be highly specific and sensitive method in detecting MAP (Kaur et al., 2011). In the present study faecal PCR detected more number of positive animals than blood PCR in LRIC farm because sheep were suffering from active clinical disease. But in early and subclinical stages of infection blood PCR was rapid, sensitive and specific test (Singh et al., 2010). Low positivity in blood PCR might due to presence of MAP in peripheral blood circulation for a limited period. This is in accordance with Singh et al. (2014) who found that 14.2% faecal PCR positive cases and 8.4% of blood PCR positive cases in Kodaikanal area of Tamil Nadu. In a similar study by Chaudhary et al. (2012) who reported 70.0% positive fecal PCR cases in Lahore district of Pakistan. Difference in the findings might be due to the intermittent shedding of the MAP bacilli in the faeces or the presence of PCR inhibitors in the faecal samples.
In sheep of Dangur farm faecal PCR detected more number of positive animals than blood PCR. Reasons for the low positive finding in the present study might be due to the sub-clinical stage or due to the lower level of infection. Low shedders were not detected by blood PCR. These findings were in agreement with Singh et al. (2013) and Coelho et al. (2008) who reported the higher prevalence (20.7%) of MAP infection in pooled samples from apparently healthy and 16.7% in blood PCR in pooled samples from suspected sheep.
However, multiple diagnostic tests are useful for the screening of JD infection in domestic livestock of the country. Since no single test is sufficient to identify all the infected animals in a flock at a given time therefore, use of multiple tests have been advocated by various workers (Singh et al., 2014; Collins et al., 2005) for the diagnosis of chronic diseases like JD.
Different test combinations were evaluated on different set of samples to formulate best strategy for the screening of sheep flock. In the present study Microscopy, when used in combination with ELISA was able to detect higher number of positive animals that were infected in comparison to the combined use of Microscopy - faecal PCR and ELISA- blood PCR. Reason being serum ELISA was highly sensitive for detection of MAP in goats, sheep, and cattle (Singh et al., 2014).
Kappa statistics and calculation of agreement between ELISA-blood PCR and microscopy- faecal PCR showed “substantial agreement” followed by moderate agreement between direct microscopy- ELISA for the detection of MAP infection in different number of samples. The present findings were in agreement with Garg et al. (2015) who also reported good agreement between serum ELISA and faecal PCR and fair agreement between microscopy and faecal PCR in dairy animals. Whereas, Singh et al. (2013) reported substantial agreement between “microscopy” and blood PCR. Microscopy and ELISA can also be a good combination to detect MAP in clinical specimen (Singh et al., 2013)
In test combinations, sensitivity and specificity of ELISA-blood PCR was higher followed by microscopy- faecal PCR and microscopy-ELISA. Sensitivity depends on the stages of disease within the flock and the antigens used in the serologic test (Hilbink et al., 1994). Present findings were in accordance with Garg et al. (2015) who also reported higher sensitivity of ELISA-PCR compared to microscopy-PCR.
Higher relative sensitivity of serum ELISA with respect to blood PCR and of microscopy with respect to faecal PCR showed that microscopy and ELISA followed by PCR were the reliable tests for the detection of MAP. As a general rule of thumb, a test with at least 95.0% specificity and 75.0% sensitivity to be used in diagnosis of disease (Pfeiffer, 1998).
Present study indicated that high prevalence of JD in Bannur breed of sheep. Periodical screening is required for confirmatory diagnosis and among the combination of tests microscopy, ELISA followed by PCR were reliable tests for the detection of MAP infection in sheep.
Conclusion
Prevalence of JD in Bannur sheep was 60.0, 76.0, and 35.2% at LRIC and 10.0, 14.0 and 50.0% at Dangur farm by microscopy, ELISA and PCR respectively. Overall prevalence was 54.7 and 16.0% in LRIC and Dangur farms, respectively using multiple tests. Amongst combination of tests microscopy and ELISA were ideal tests for screening of JD in the flock, and PCR was best utilized for confirmation of individual cases. Variable clinical symptoms weakness without diarrhoea in Ovine JD, multistages disease and the zoonotic importance of MAP infection demand immediate attention for control. Screening of all the animals before introducing in to the flock with multiple tests should be mandatory. Utility of multiple diagnostic tests is suggested for confirmatory detection and epidemiological diseases investigations of MAP in animals. In addition, immediate implementation of control programmes for JD both at State and National level is need of the hour.
Acknowledgements
Authors are thankful to Indian Council of Agricultural Research (ICAR) and Karnataka Veterinary Animal and Fisheries Sciences University (KVAFSU), for provision of funding and facilities through ICAR -NAE project ‘Animal Disease Registry and Tissue Bank’, Department of Veterinary Pathology, Veterinary College, Hebbal, Bengaluru.
Conflict of Interest
We declare that we have no conflict of interest.
Author’s Contribution
All authors contributed equally.
References








