Advances in Animal and Veterinary Sciences
Research Article
Following-Up the Salmonella spp. Carrier Status (Contamination) from Piglet to Carcass
Cocora Zoriţa Maria1*, Cziszter Ludovic Toma2, Ţibru Ioan1
1Faculty of Veterinary Medicine; 2Faculty of Animal Science and Biotechnology, Banat’s University of Agriculture and Veterinary Medicine “King Michael I of Romania” from Timisoara (BUAVMT), 119 Calea Aradului, Timisoara 300645, Romania.
Abstract | The aim of this paper was to identify the critical points on the technological flow of pig rearing in order to reduce Salmonella spp. contamination. Three farms (A, B, and C) were taken into study, where Salmonella spp. carrier status was determined on five series of 10 sows each together with their piglets from farrowing to slaughtering in farms A and B, and only from nursery to slaughtering in farm C. A total of 450 faecal samples were collected from farms and 100 sanitation samples were collected from carcasses on the technological flow in slaughterhouse. Samples were analysed using the classical method SR EN ISO 6579 / 2003, and PCR method, and then were sent to sequencing. The most frequent isolated serovars were S. typhimurium, S. enteritidis, S. typhi and S. agona. Results showed that in farm A 50% of the samples were positive both in sows and in piglets, while in farm B the incidence was 33% for sows and 27% for piglets. In young stock, the positive samples increased up to 57% in farm A and to 43% in farm B. As the age of the animal increased the contamination increased, thus the percentage of positive samples was 68% in farm A, 67% in farm B and 87% in farm C. Tracing the same animals in the slaughterhouse technological flow, it was observed that the percentage of positive samples was 80% in the lairage area, 77% after stunning, 8% after scalding and singeing, 53.84% at depilation, 62.85% at eviscerating and 17% at chilling.
Keywords | Carcass, Faecal, Identification, Pigs, Salmonella spp., Serovars
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | March 05, 2015; Revised | March 31, 2015; Accepted | April 02, 2015; Published | April 09, 2015
*Correspondence | Cocora Zoriţa Maria, Banat’s University of Agriculture and Veterinary Medicine “King Michael I of Romania” from Timisoara, Calea Aradului, Timisoara, Romania; Email: [email protected]
Citation | Maria CZ, Toma CL, Ioan Ţ (2015). Following-up the Salmonella spp. carrier status (contamination) from piglet to carcass. Adv. Anim. Vet. Sci. 3(5): 283-288.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.5.283.288
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Maria et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Salmonella is the most common cause of food poisoning in humans (Wegener et al., 2003), and in Europe, based on existing information, in the last 20 years (Schlundt et al., 2004), it was found that Salmonella remained the second etiologic agent of zoonoses, despite its declining incidence in recent years (EFSA, 2010). Therefore in the present study, we wanted to know the carrier status and the degree of carcass contamination on the technological flow, to reduce the spread of contamination, condemnations and economic losses. Due to the high incidence of Salmonella spp., and the fact that most cases occur after consumption of pork products (Fedorka-Cray et al., 2000), it is essential to consider each stage of the tehnological flow to prevent contamination of pork (Schlundt et al., 2004). Within this context the purpose of our research isfoundthe purpose of our research.
Pigs shedding Salmonella, without clinical signs, are those that create problems in public health because it can contribute to contamination of the finished product (Malorny and Hoorfar, 2005).
Knowing the vulnerabilities we can take measures to reduce contamination due to carrier status, thereby increasing the chances of getting more Salmonella spp.-free carcasses.
MATERIALS AND METHODS
The study was conducted in three pig production farms, in which, carrier status was monitored from gestation to fattening (farm A, B), and in the third farm (C), only from nursery to slaughter.
In the first stage, faecal samples were collected from five groups of 10 sows each (May 2014 - January 2015), and their piglets. In the second phase (farm C), faecal samples (50) were collected from nursery to slaughter (September-December 2014).
The selection of the farms was made according to the duration of the transport from the farm to the slaughterhouse. Farm A, had the shortest transport, farm B, the longest and farm C was in the middle. The batches of pigs (A, B) and piglets (farm C) were randomly selected.
It was decided to determine the carrier status because the results obtained by this method (microbiological), even if they are specific to Salmonella identification, gives to the slaughterhouse DVM a timely information about the epidemiological situation of the livestock that will be slaughtered, instead of the serological method who gives a history of the epidemiological situation of the livestock that arrived at the slaughterhouse.
Pigs were slaughetred at the same slaughterhouse, where 100 samples were collected (50 per farm A and B). The samples were processed in accordance with SR EN ISO 17604 / 2003 (four swabs for each carcass) starting from the reception (faecal sample), stunning, scalding, depilation, singeing, evisceration, and refrigeration. The 50 samples collected from the farm are in accordance with the number of animals brought for slaughter, under 2000 heads/day.
In parallel, 50 sanitation samples were collected from the working equipment or machinery.
Processing of samples was carried out in accordance with SR EN ISO 6579/2003 / AC: 2009 and some of the positive samples were analyzed by PCR, and then after isolation and identification were sent to sequencing in Netherlans (Macrogene Euro®, Amsterdam, Netherlands Laboratory).
Preparing the Samples
For the pre-enrichment step, 25 g faeces were mixed with 225 ml buffer peptone water (APT) and incubated at 37 ± 1°C for 18 ± 2 hours. Sanitation samples from the surface of carcasses were collected using sterile swabs and placed in peptone water container. After incubation, the APT culture was inoculated by using a loop, on agar plate in order to achieve the molecular reaction. For the enrichment stage of the culture 0.1 ml of APT were transferred into a test tube together with 10 ml Rapaport-Vassiliadis (RVS) and incubated at 41.5 ± 1°C for 24 ± 3 hours, and 1 ml APT was transferred into a 10 ml Muller-Kauffmann (MKTTn) tube for 24 ± 3 hours at 37 ± 1°C.
After incubation, both the culture obtained in RVS broth and MKTTn broth, were inoculated on one plate with xylose-lysine-desoxycholate (XLD) solid medium and one plate with Rambach solid medium, having in the end four plates for each sample.
From the culture obtained on the RVS was inoculated one agar plate, followed by a period of incubation at 37 ± 1°C for 24 ± 3 hours.
After the time expired the plates were read, and those with specific colonies were selected for biochemistry confirmation.
Extraction of DNA using DNA Mini Kits PureLinkTMGenomic
For the DNA extraction the following steps were folowed: decimal dilutions were performed from the agar culture, so we obtained a load of 2 x 109 cfu. A part of the culture and 1000 µl sterile saline was placed in the ependorf tubules. The sample was centrifuged for 1 minute at 8000 rpm to give a residue, then the supernatant was removed from each tubule. PureLinkTMGenomic Digestion Buffer was addedin the amount of 180 µl, then 20 µl proteinase K, which causes the bacterial cell wall lysis, determining the DNA release, followed by homogenization. Then 20 µl of RNase A was added followed by another homogenization, and then 200 µl Pure LinkTMGenomic Lysis / Binding Buffer was added. Ethanol (200 µl, 96-100%) was added and was homogenized for 5 seconds until a homogeneous solution was obtained; DNA obtained was 15-60 mg, but depending on the sample and the manner of storage can reach from 5 to 100 mg. We kept it at -20°C.
Polymerase Chain Reaction
PCR was performed by the technique described by Castagna et al. (2005) and Ohud et al. (2012) with some modifications.
Gene Amplification
The actual amplification was performed by classic PCR, in one stage and was based on creating multiple copies of a sequence of Salmonella gene inv A of 284-bp size using the inv A-139 primer (5’-GTGAAATCGCCACGTTCGGGCAA-3 ‘) and reverse inv A-141 (5’-TCATCGCACCGTCAAAGGAACC-3 ‘).
A Master Mix MyTaqTMRed Mix (BIOLINE®) containing the components required in the reaction in a 2 x concentrated form was used for carrying out the reaction. The reaction volume was 25μl, of which: 12.5 MyTaqTMRed Mix (BIOLINE®), 1 µl Inv A, 1 µl Inv R, 2 µl DNA, and 8.5 µL H2O.
Amplification was performed through the Bio-Rad thermocycler My Cycler System (where the distortion of the DNA molecule, primer annealing and elongation in several successive cycles took place).
This program included the following steps: a cycle of DNA denaturation at 95°C for 1 minute; 32 cycles of: denaturation at 95°C for 30 seconds, hybridization at 55°C for 30 seconds, and extension at 72°C for 30 sec; incubation at 4°C.
Control of the amplicons was carried out by horizontal electrophoresis in 1.5% agarose gel at 120 volts and 90 mA for 60 minutes. After migration of the samples, their reading was performed using a Photo Doc-It Imaging System camera. After the DNA purification and verifying the presence of DNA by its migration on the agarose gel, positive samples were sent for sequencing.
The statistical analysis consisted in comparig the positive and negative samples using the chi square test in Statistica software (StatSoft, 2013).
RESULTS
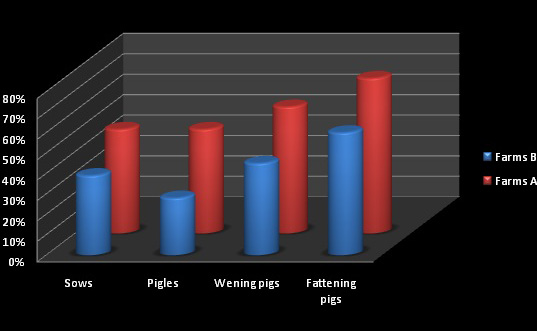
The prevalence of Salmonella spp. after faecal samples examination from each farm, revealed the following: in farm A (n = 200), in maternity 50% of samples were positive both in sows and piglets; after weaning an increase of 57% was noticed, and before the delivery to the slaughterhouse prevalence reached 75%. In farm B 33% positive sows and 27% piglets were found in maternity, after weaning prevalence reached 43% and before slaughtering 67% were positive samples (Figure 1).
The fact that in farm A, the number of positive samples were equal in both, sows and piglets, leads to the conclusion that sows manage to transmit the carrier status to their piglets, although none showed clinical signs. This is a disadvantage, because weaning and then transportation are stress factors that may favour the onset of the disease.
In support of this idea are the results from farm B, where less positive samples allowed the maintenance of the carrier status at lower levels. The fact that prior the transportation to the slaughterhouse, in farm A were 75% positive samples, compared to 67% in farm B, allows us to conclude that there was a cross-contamination between weaned piglets, but is directly proportional to the number of positives piglets arrived at fattening.
Following statistical interpretation of the results obtained on the farm A, non-significant differences were found (p > 0.05) between sows and piglets, significant differences (p < 0.01) between sows and youth, and highly significant differences (p < 0.001) between sows and fattening pigs.
Doing the same statistical interpretation in farm B significant differences (p < 0.01) were found between sows and piglets, highly significant differences (p < 0.001) were observed between sows and fattening pigs, and between young stock and pigs that reached the age of slaughter. Non-significant differences (p > 0.05) were found between sows and young stock.
In C farm, where the pigs were gathered from many different farms for fattening, the load with Salmonella spp. (of the digestive tube) was 87%, significantly higher (p < 0.01) than in the other two farms.
The obtained results were similar to those in the literature, which showed the role of sows as a potential source of infection for the infant piglets (Davies et al., 1998; Funk et al., 2001; Letellier et al., 1999).
Contamination after weaning is due to increased susceptibility to infection with Salmonella spp. because of: weaning stress, reduced immunity, sudden change of regime and mixing piglets (Funk et al., 2001; Kranker et al., 2003; Nollet et al., 2004; Van de Ligt et al., 2002).
In the slaughterhouse, after analysing the results obtained from the slaughtered pigs (n = 100), there was a variation of the Salmonella spp. load, specific to each step of the process flow, as follows:
The fact that after the stabbing, the carrier status decreased by 3% can be attributed to the compliance of the showering stage; the reduction to 10% positive samples, after scalding is because the scalding time and water temperature was respected. The waxing phase due to the epilator movements that help remove faeces of rectal ampulla, determined an increased prevalence to 54%. In evisceration stage, because specific actions and the inconsistency of the best practice procedures by staff each time positive samples reached 63% value. Through the specific stages of final showering and rapid cooling the number of positive samples was significantly reduced.
In farm A, analysing the results from samples taken from the lairage area, where all samples were positive (100%), after stunning, there was a reduction to 88% due to the pigs washing before slaughter, after scalding the results were 0% positive, so that after depilation stage to increase to 25%, and to reach 60% after evisceration and 12.5% at chilling step.
As a result of samples analysed taken from pigs originating from B farm 90% positive samples were obtained in the lairage area, 60% after stunning, 0% after scalding and after the hair removal and evisceration step the number of positive samples increased to 80%, and in the last stage (refrigeration) decreased to 10%.
Statistical interpretation of the results showed significant differences (p < 0.001) between the lairage area, scalding and chilling of carcasses; distinctly significant (p < 0.01) between the lairage area and polishing, respectively significant differences (p < 0.05) between lairage and evisceration area, and between the lairage area, waxing and stabbing no differences were found (p > 0.05).
Between stabbing, scalding, polishing and chilling of the carcasses significant differences (p < 0.001) were noticed, distinctive significant differences (p < 0.01) between stunning and eviscerating, and between stunning and depilation non-significant differences (p > 0.05) were found.
Between scalding, waxing, polishing and dressing statistically significant differences (p < 0.001) were found and non-significant differences (p > 0.05) between scalding and chilling.
There were significant differences (p < 0.001) between hair removal, evisceration and chilling, distinctive significant differences (p < 0.01) between polishing and refrigeration, and between waxing, polishing and dressing non-significant differences (p > 0.05) were found.
In the statistical interpretation from farm B non-significant differences (p > 0.005) were found between the waiting area and stunning, scalding, depilation, and before the chilling of carcasses, but distinctive significant differences (p < 0.01) between scalding, depilation, polishing, and evisceration were found.
DISCUSSION
Following the summarization of results obtained from the two farms and presented above, we observed a correlation with data from the literature, of traceability of Salmonella spp. from the sows to the carcass. Farm-level analyses showed that the load of Salmonella in pigs before delivery, depends on the degree of contamination of sows in maternity, which can lead to contamination of piglets at this stage.
Different values were observed, specific to the farm, so in the farm A, where the percentage of sows was equal with piglets, support the idea of transmission from the sow to piglet. The fact that after weaning in both farms, there was an increase in the degree of contamination can be blamed on contamination between piglets grouped on bodyweight in finishing pens.
Further analysing of groups of pigs in the study, arrived at the slaughterhouse in the lairage area, there was a significant increase in the level of contamination; on the A farm reaching 100% positive samples. This is due to lack of sanitation of the pens in the lairage area between batches.
As already mentioned, the lairage area is a source of contamination with Salmonella spp., mentioned in the literature, because the risk of contamination of the pigs free of Salmonella spp. is increased with increasing lairage time (EFSA, 2010).
The scalding by immersing the carcasses in a water tank (62°C for 8 minutes) determines the loss of the majority of micro-organisms on its surface (Koutsoumanis et al., 2009). Survival of Salmonella during scalding phase occurs when the water temperature is not respected, only if it drops below 62°C (Hald et al., 2003) and/or where the amount of organic material is sufficiently high causing the protection of Salmonella against the heat (Sörqvist et al., 1990).
Depilation, the mechanical removal of hair, is a source of carcass contamination with faeces (Borch et al., 1996). Da Silva et al. (2003) in a study, was noticed the increased number of positive carcasses with Salmonella spp. after the depilation stage compared with evisceration stage.
The high degree of contamination of carcasses before singeing was associated with the failure of the washing stage of pigs before slaughter (Letellier et al., 2009), the contamination of the water (Hald et al., 2003) or the depilation process itself (Pearce et al., 2004).
The polishing step determined the recontamination of the carcasses according to Rivas et al. (2000) and Yu et al. (1999), while Gill and Bryant (1992) found decreased levels of contamination during this stage. Contamination of carcasses during the next stages of the technological flow may be due to failure of cleaning stages of working equipment (Borch et al., 1996), out of which the water temperature at washing is crucial (Sadeleer at al., 2008).
During the process of evisceration, a contamination of carcasses occurs up to 55-90% (Berends et al., 1997). Dietary compliance of animals before slaughter, correlated with the compliance of evisceration reduces the risk of carcasses contamination.
Botteldoorn et al. (2003) studied the carcasses from three slaughterhouses and found that the contamination was different. At one slaughterhouse Salmonella spp. wasn’t isolated from the surface of the carcass, versus another slaughterhouse were 36% were positive samples for Salmonella spp. In two slaughterhouses studied by Botteldoorn et al. (2003) the Salmonella contamination levels decreased due to refrigeration stage. Mafu et al. (1989) found an increased prevalence of Salmonella spp. (12.5%) on the pavement surface of the refrigerated warehouse, which was assigned to the personnel “comes and goes”.
After analysing the samples sent to the sequencing and put them in the bank gene (GeneBank), it was found that the most common serovars isolated from faeces were S. typhimurium DT104 and S. newport and on the surface of carcasses, on the tehnological flow in the slaughterhouse most frequently isolated serovars were S. newport, S. typhy, S. enteritidis and S. typhimurium var. 5.
Analysing the results from the samples taken from the surface of working equipment or machinery, it was found 36% positive samples, and after typification serovars S. typhimurium and S. agona were isolated.
The results obtained in the present study are in according with those obtained by EFSA (2008), in 2006-2007, on a total of 387 cases regarding the most frequent isolated serovars in the Member States, S. typhimurium was the most frequently serovar identified on the surface of pig carcasses, representing 49.4%, followed by S. derby (24.3%), followed by S. infantis, S. bredeney and S. brandenburg (3.4%, 2.1% and 1.8%).
S. typhimurium was the most frequently isolated serovar in 10 Member States, S. Derby was the second serovar isolated in seven Member States, Belgium, Czech Republic, Denmark, France, Ireland, Latvia and the United Kingdom (EFSA, 2008).
In conclusion, Salmonella carrier status (by sampling of feces) was equal to piglets and sows in farm A (53%), but different in farm B where sows was 39% and piglets 28%.
During the rearing (fattening) process, there was an increase in the carrier status, statistically similar in all the three farms.
Comparing the last values before transport, with those from the reception at the slaughterhouse, there was an increase in the degree of contamination (transport stress).
During slaughtering process, analysing the samples from the surface of carcasses, significant differences between the control points from the dirty area and the clean area were found, so it was found a high degree of contamination in the clean area, in the evisceration stage, decreasing significantly in the chilling stage of the carcass, which inhibits the growth of microorganisms of the genus Salmonella.
The most common serovars isolated by PCR were S. typhimurium, S. newport, S. typhi, S. enteritidis and S. agona.
ACKNOWLEDGEMENTS
This paper was published under the frame of European Social Fund, Human Resources Development Operational Programme 2007-2013, project no. POSDRU/159/1.5/S/132765.
CONFLICT OF INTEREST
The authors declare that they have not conflict of interest.
REFERENCES