Advances in Animal and Veterinary Sciences
Review Article
Concise Review: Appreciated Signaling Role of High Mobility Group–A Proteins for Regulation of Proliferation, Pluripotency and Self-Renewal of Adult Stem Cells
Ahmed Abdelbaset Ismail
Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Sharkia, Egypt.
Abstract | The potential application of pluripotent stem cells and retaining their pluripotency without tumour formation in regenerative medicine is very beneficial and challenging. It is really imperative to capture which environmental niche keys make these cells steadily growing and retaining their self-renewal capacity, particularly post–implantation. Based on the results extracted from our and other studies, high mobility group proteins-A (hmga), particularly hmga2, could fulfil that function very properly through suppression of cell proliferation inhibitors and maintaining/enhancing the genes responsible for stem cell pluripotency such as lin28, klf4, sox2, cmyc, oct4, rex1, sall4, smad3/4, goosecoid (gsc), brachury, nanog and utf1. Based on these observations, we provide evidence that hmga2 protein is an appealing candidate that could be employed in stem cell translational regenerative medicine to achieve that notion. This review states brief background of hmga pathway function with spotlight on the pre-clinical literature that links hmga signaling to adult stem cells proliferation and self-renewal.
Keywords | Self-renewal, Pluripotency, Stem cells, hmga, Proliferation
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | March 17, 2015; Revised | March 29, 2015; Accepted | March 30, 2015; Published | April 08, 2015
*Correspondence | Ahmed Abdelbaset Ismail, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Sharkia, Egypt; Email: [email protected]
Citation | Ismail AA (2015). Concise review: appreciated signaling role of high mobility group–a proteins for regulation of proliferation, pluripotency and self-renewal of adult stem cells. Adv. Anim. Vet. Sci. 3(5): 276-282.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.5.276.282
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Ismail. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Stem cells were primarily studied by Becker’s group, when they observed development of splenic nodules after inoculation of bone marrow mononuclear cells into irradiated mice. The number of these formed nodules was in proportion to the number of injected cells. Analysis of those cells afterwards revealed that those cells have the capacity of self-renewal and potentiality to give rise to several specialized cell types (Becker et al., 1963).
Therefore, the capability of self-renewal, repopulation to other stem cells and progenitors, and to giving rise to various committed cell lineages under special conditions are by definition the three essential properties of stem cells.
Stem cells can generally be categorized into two main types, embryonic and non-embryonic. Embryonic stem cells (ESCs) are derived from blastocyst and have the features of totipotency, thus could progenerate into all cells of embryonic germ layers, while non-embryonic stem cells (non-ESCs), also termed adult stem cells, are multipotent and could propagate into several kinds of tissue committed cells, however this appears to be more finite (Lee and Hui, 2006).
Recent perception proposed that the adult tissues contain primitive pluripotent stem cells (Kucia et al., 2007; Santourlidis et al., 2011; Roger et al., 2012; Ogura et al., 2014). The employment of these recent identified adult pluripotent stem cells potentially for organs regeneration needs to address the biological keys [i.e., high mobility group proteins–A (hmga)], that play a crucial role in maintaining the self-renewal and pluripotency features of these cells especially after theirimplantation (Ismail et al., 2012).
In this review we give a brief outlook on stem cells types and stem cell niche with focusing on hmga proteins.
STEM CELL NICHE
A niche or stem cell microenvironment is composed of signaling molecules, cell–cell interaction and neighbouring extracellular matrix (ECM). This three structured dimensional niche so far is considered in jurisdiction of stem cells genes and hence disciplines their stemness properties, e.g., proliferation, self-renewal, migration, homing and commitment to other cell types (Watt and Hogan, 2000).
At special circumstances, single signalling pathway generated from this niche is appropriate for enhancing the self-renewal capacity. For instance, in germ line stem cells (GSCs) derived from the Drosophila female, bone morphogenetic protein (bmp) is sufficient for their self-renewal (Xie, 2013), and Notch for C. elegans GSC self-renewal (Byrd and Kimble, 2009). Moreover, activation of Notch1 leads to increasing the haematopoietic stem cells (HSC) self-renewal capacity (Kunisato et al., 2003).
On the other hand, most stem cell types are simultaneously activated by several pathways for maintaining long term stem cell self-renewal, e.g., sonic hedgehog (Shh), fibroblast growth factor (FGF) and brain-derived neurotropic factor (BDNF) signalling pathways are essential for neural stem cells (NSC) (Zhao et al., 2008). Furthermore, there are many cell surface molecules having a critical role in either cell – cell or cell – ECM adhesion in addition to self–renewal of stem cells. From these molecules, cadherin and integrin (α, β) molecules which are observed expressed in a large scale of adult stem cells (Chen et al., 2013).
PLURIPOTENT STEM CELL TYPES
Pluripotent Embryonic Derived Stem Cells
During the earliest period of embryo formation, the inner cell mass (ICM) of blastocyst “origin of embryonic stem (ES) cells” evolves into two separate germ cell layers, epiblast and hypoblast. The hypoblast cells are responsible for formation of yolk sac “origin of the primitive hemangioblasts”, and the epiblast forms embryonic tissue layers, i.e. ectoderm, mesoderm and endoderm (Martin, 1981). Consequently, Thomson’ group succeeded to isolate these cells and approved the first human ES cell line in 1998 (Thomson et al., 1998). However, there are many concerns regarding the use of either ES cells or induced pluripotent stem (iPS) cells such as teratomas formation as result of their unlimited proliferation, genetic manipulation, high rates of immunorejection, and ethical concerns (Przyborski, 2005).
Non-embryonic Pluripotent Stem Cells
Mesenchymal stem cells, especially which isolated from adipose tissue, adipose mesenchymal stem cells (ASCs) showed very promising results and considered as alternative multipotent stem cell source to mesenchymal stem cells isolated from bone marrow (BM–MSCs) (Zuk et al., 2001).
Similar to BM–MSCs, ASCs have previously been confirmed for the capability of multilineage differentiation; e.g., a chondrogenic (Neupane et al., 2008; Zuk et al., 2001), osteogenic (Lo et al., 2012; Levi et al., 2010), adipogenic (Neupane et al., 2008; Zuk et al., 2001), neurogenic (Lin et al., 2006; Safford et al., 2002), myogenic (Wang et al., 2014; Chen et al., 2004), angiogenic (Tang et al., 2004), and cardiomyogenic (Gwak et al., 2009) lineages.
There are however some specific advantageous criteria make the use of ASCs as rich sources for adult stem cells very appealing. Obtaining sufficient amount of adipose tissue samples using lowest levels of invasion and pain in addition to high capability of ASCs to retain their multipotency and phenotype after long term of culturing reach to 25 passages make these cells very attractive (Liu et al., 2007; Zhu et al., 2008).
Following, recent pluripotent stem cell populations have been identified and raised the consideration of the scientific community as a valuable alternative candidate to the argumentative ES cells and genetically reprogrammed iPS cells. From these cells, multipotent adult progenitor cells (MAPCs) (Jiang et al., 2002), human marrow-isolated adult multilineage inducible (MIAMI) cells, unrestricted somatic stem cells (USSCs), very small embryonic-like stem cells (VSELs), stimulus-triggered acquisition of pluripotency (STAP), and multilineage differentiating stress enduring (Muse) cells are well known. MAPCs isolated from bone marrow, have been shown to regenerate the infarcted myocardium in mouse model (Dimomeletis et al., 2010). VSELs, MIAMI and USSCs were isolated from bone marrow and cord blood and showed pluripotency and non tumorgenic properties (Kucia et al., 2007; Santourlidis et al., 2011; Roger et al., 2012).
In addition to other regenerative capacities of VSEL in vivo, more recent published paper has showed very promising neovasculogenic capacity of VSELs in patients suffering from critical limb ischemia (Guerin et al., 2015).
In 2010, Muse cells have been identified from adherent mesenchymal stromal cells isolated from bone marrow and lipoaspirats and also from commercially available human adipose stem cells (Muse–AT cells). This population of cells showed expression of pluripotency marker stage-specific embryonic antigen-3 (ssea-3) as well as the double expression of mesenchymal cell specific markers CD105 and CD90. However, as declared by Simerman et al. and others, Muse cells displayed only a slight increase in expression of pluripotency markers (sox2, naong, oct4) when compared with non-Muse cells (Kuroda et al., 2010; Wakao et al., 2011; Ogura et al., 2014; Simerman et al., 2014).
LINK BETWEEN HMGA AND ADULT STEM CELLS SELF-RENEWAL
Pluripotency Gene Expression
Pluripotency means the tremendous capacity of stem cells to differentiate into all types of cells specified from all embryonic germ layers. Studies of the relationship between pluripotency and gene expression led to the identification of variety of markers specific for pluripotent stem cells in mammals. These transcription factors including oct3/4 (Rizzino, 2009), sox2, nanog (Mitsui et al., 2003), cmyc (Smith et al., 2010), rex-1 (Ben-Shushan et al., 1998), tra-1 (Andrews et al., 1984) and klf4 (Chan et al., 2009), have been shown to contribute to the long-term maintenance of an embryonic stem (ES) cell-like phenotype, proliferation and maintenance as well as self-renewal of pluripotent cells. Currently, ssea–3 and –4 are commonly used as surface markers to prove the pluripotency of human stem cells (Yang et al., 2013b; Tsai et al., 2013; Bouwens, 2012; Yang et al., 2013a; Truong et al., 2011; Holford et al., 1994). Ssea-3 and ssea-4 are globoseries glycosphingolipid surface epitopes and their expression indicates that the ES cells have increased the levels of a metabolic activity, especially during early embryogenesis (Suila et al., 2011).
The concept of retaining the pluripotency and self–renewal potentiality of stem cells, especially post transplantation in vivo is really critical. For this reason, the characterization of signaling molecules governing that notion of regular proliferation and self-renewal in steady states is really essential for both in vitro and in vivo studies. Some recent studies have focused on that subject and based on their in vitro and in vivo data, they proposed that “hmga” can influence and actively regulate these actions (Nishino et al., 2013; Ismail et al., 2012; Monzen et al., 2008; Nishino et al., 2008; Sanna et al., 2008).
High Mobility Group–A (HMGA) Proteins
Hmga proteins (formerly known as HMGI/Y) are category of large unique nuclear non-histone chromosomal architectural proteins (Grosschedl et al., 1994). AT–rich DNA nucleotide sequences with acidic C-terminus (Reeves, 2000) are the common structural and active motifs in encoded genes of those proteins. This type of unique proteins is specialized into two distinguished encoded genes, hmga1 and hmga2 (Friedmann et al., 1993; Johnson et al., 1989).
Importance of HMGA Proteins Expression
Initially, has been approved that hmga1 is important for cell differentiation, while hmga2 is commonly involved during stem cells proliferation and self–renewal (Vartiainen et al., 1988).
These data were afterwards confirmed by another group who declared that up regulation of these unique proteins in unspecialized and primitive stem cells isolated from embryos revealed the crucial role of hmga proteins in management of stem cell differentiation and proliferation. Furthermore, it has been ascertained that hmga2 is expressed by embryonic stem cells isolated from either murine or human blastocyst (Tay et al., 2009). All these data provide evidence of the very crucial role of hmga protein during embryonic and foetal growth and development.
HMGA Proteins and Pluripotency Genes
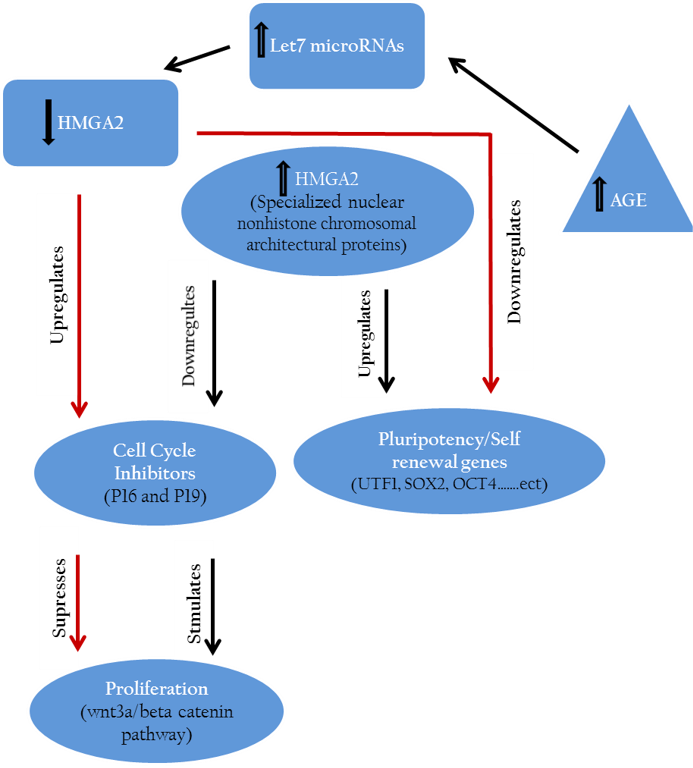
The pathways link hmga2 to other some cellular genes that control self–renewal, proliferation and pluripotency are displayed in Figure 1. Expression experiments performed on human ES cells at mRNA levels indicated that there is a clear correlation between the expression level of hmga2 and pluripotency genes including utf1, sox2 and oct4 (Li et al., 2007).
Hmga2 controls the regulation of cell growth inhibitors (P16, P19) and accordingly maintains or accelerates the proliferation of stem cells. On the other side hmga2 plays a crucial role in up regulation of pluripotency specific genes hence retains the pluripotency features of stem cells.
Further, hmga2 were down–regulated through let-7 microRNAs up-regulation pathway after inducing germline derived cancer cell line e.g., murine P19 teratocarcinoma cell line toward neuronal lineage (Eda et al., 2009).
Similarly, down regulation of proliferation inhibitors including let-7b, p19 (arf) and p16 (ink4a) mediated by hmga2 expression led to maintenance of mouse neural stem cells phenotype in vitro (Nishino et al., 2008). Other results indicated the suppressing effect of down regulated cmyc and hmga1 expression on proliferation of stomach cancer cell line through wnt3a/beta-catenin signaling pathway (Akaboshi et al., 2009).
Furthermore, at a molecular level, our previous study has demonstrated that relative expression of pluripoteny specific genes hmga2, oct4, klf4, sox2 and cmyc were maintained after in vitro stimulation of canine adipose mesenchymal stem cells (cASCs) with recombinant human hmga2 protein (Ismail et al., 2012) as depicted in Figure 2.
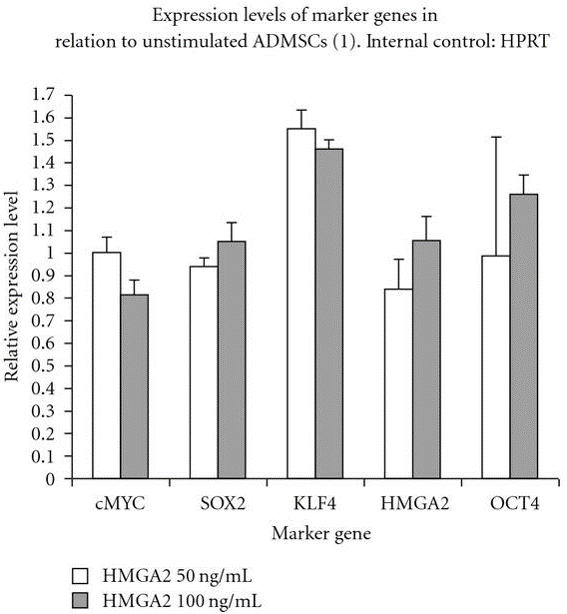
Figure 2: Relative pluripotency genes expression by stimulated and unstimulated canine adipose mesenchymal stem cells (cASCs) with hmga2 protein (50, 100 ng/ml) after real time PCR
OCT4, KLF4, cMYC, HMGA2 and SOX2 are expressed by cASCs but no difference was detected at the used concentrations.
Role of HMGA Proteins in Cell Proliferation
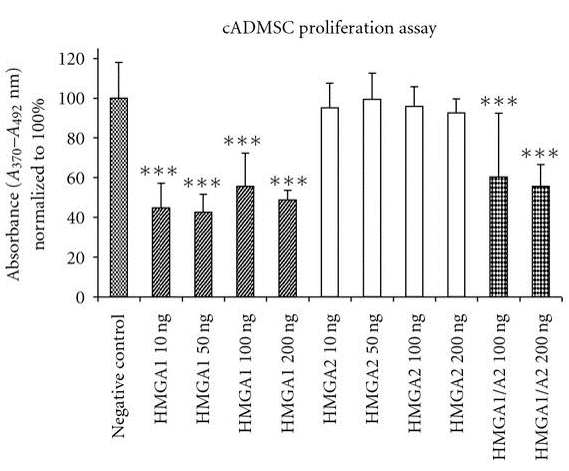
The role of hmga proteins on proliferation of undifferentiated ES cells and tissue specified cells have been proposed by several studies (Pierantoni et al., 2003; Caron et al., 2005; Li et al., 2007; Richter et al., 2009; Ismail et al., 2012). The action of hmga1 protein has been assessed for accelerating enhanced adipocyte proliferation and expansion of human fat derived tumours (Pierantoni et al., 2003). It has also been demonstrated the critical regulatory function of hmga2 in skeletal muscles formation from murine ES cells (Caron et al., 2005) and adipogenic lineage differentiation of MSCs (Li et al., 2007). Additionally, porcine chondrocytes showed significant increased proliferation in vitro, when incubated with hmga1 and hmga2 in different supra–physiological concentrations (Richter et al., 2009). In consistently, hmga1 significantly did reduce cASCs proliferation, when employed alone or in combination with hmga2, however there was no enhanced or inhibiting effect observed after cultivation of cASCs with hmga2 protein in vitro (Figure 3) (Ismail et al., 2012). More recent study revealed that lin28b with hmga2 may act as a regulator of HSC self-renewal potential (Copley et al., 2013). Furthermore, a network of hetero-chronic gene products including lin28a, let-7, imp1, and hmga2 have a vital role in regulation of neural stem cell characteristics.
Hmga1 has significant inhibiting effect on cell proliferation when used alone (10–200 ng/ml) or combined with hmga2 (100, 200 ng/ml). Contrarily, there is no significant differences in cell proliferation are observed in hmga2-treated groups compared to un-stimulated cells. One-way analysis of variance and Tukey test was performed for statistical comparison (significance levels: *P<0.05; ** P<0.01; *** P<0.001).
CONCLUSION
Pluripotency and self-renewal are overwhelmingly considered the most essential properties of adult pluripotent stem cells. These features are regulated by unique stem cell specific transcription factors, namely pluripotency genes. These factors include lin28, klf4, sox2, cmyc, oct4, rex1, sall4, smad3/4, goosecoid (gsc), brachury, nanog and utf1. To retain the self-renewal capacity of either transplanted or cultivated pluripotent stem cells, the balances between these transcription factors and other signaling controlling molecules are fully critical and needed to be elucidated. Thereby, the identification of the biological keys that control that notion is very imperative for both in vitro and in vivo strategies. Recent studies performed by us and others proposed that hmga proteins have the potentiality to govern those functions via direct or indirect regulation of other cellular processes – controlling genes.
ACKNOWLEDGEMENTS
This work was supported by Faculty of Veterinary Medicine, Zagazig University and Ministry of Higher Education, Egypt.
CONFLICT OF INTEREST
There is no conflict of interest
References