Advances in Animal and Veterinary Sciences
Short Communication
Hemato-biochemical and Serum Iron Profile Study in Dogs with Hemoprotozoal Infection
Jithin Mullakkalparambil Velayudhan*, Dighe Dhananjay Govind, Velhankar Rajendra Dattatray, Keskar Dattatray Venkatesh
Department of Veterinary Clinical Medicine, Ethics and Jurisprudence, Bombay Veterinary College, Parel, Mumbai- 400012, India.
Abstract | Iron is an essential mineral for all living organisms and is integral to multiple metabolic functions. The most important function being oxygen transport in haemoglobin. The use of serum iron profile as a routine parameter is limited for assessment of anaemia in dogs. The present study was carried out in anaemic dogs with hemoprotozoal infection (n=6) to observe the type of iron metabolism disturbance that occur.. The mean ± standard error (SE) of serum iron analysts [serum iron, serum total iron binding capacity (TIBC) and percent transferrin saturation (%TSAT)] were measured by calorimetric analyser to estimate the iron profile status in anaemic dogs. Statistical significant difference (P≤ 0 .05) was found between the serum iron parameter and non-significant difference between serum TIBC and %TSAT. The alteration in iron profile observed was similar to anaemia of inflammatory disease which also provided a conclusion regarding the therapeutic approach to be followed for the occurred iron metabolism disturbance.
Keywords | Anaemia, Serum iron, Serum total iron binding capacity, Percent transferrin saturation, Dogs
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | February 02, 2015; Revised | March 05, 2015; Accepted | March 07, 2015; Published | March 12, 2015
*Correspondence | Jithin Mullakkalparambil Velayudhan, Bombay Veterinary College, Parel, Mumbai, India; Email: [email protected]
Citation | Velayudhan JM, Govind GG, Dattatray VR, Venkatesh KD (2015). Hemato-biochemical and serum iron profile study in dogs with hemoprotozoal infection. Adv. Anim. Vet. Sci. 3(4): 200-206.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.4.200.206
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Velayudhan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Iron is an essential element for all living organisms and about 60-70% of total body iron is present in hemoglobin (McCown and Specht, 2011; Harvey, 2008). Imbalance of iron in the body will result in number of clinical manifestations and which may lead to severe complications or even be life threatening (McCown and Specht, 2011). Dietary iron is absorbed from duodenal enterocytes by the apical surface (Edison et al., 2008) into the plasma where it is bounded to transferrin for transport to cells within the body (Harvey, 2008). Disruption in iron intake, loss or regulation can lead to clinical manifestations such as iron deficiency anaemia, anaemia of inflammatory disease, and iron overload (Lieu et al., 2001; McCown and Specht, 2011). Iron deficiency caused by inadequate intake is rare in dogs because most foods have adequate iron content and major cause of iron deficiency anaemia in dogs is chronic blood loss, usually in the gastrointestinal tract (e. g. tumours, gastric ulcers, inflammatory bowel disease, and parasites) (Zaldivar-Lopez et al., 2014). Monitoring the iron status of an animal is done by evaluation of serum analytes (serum iron, serum total iron binding capacity (TIBC), serum ferritin and percent transferrin saturation (%TSAT) (Wians et al., 2001) but serum ferritin concentration has limited use in veterinary medicine because reagents are species-specific and the assay is not widely available (Schaefer and Stokol, 2015). Depending on the magnitude of iron loss, anaemia may be regenerative or non-regenerative characterized by microcytic normochromic to microcytic hypochromic indices (Mitchell and Kruth, 2010). Hence alteration in serum analytes of iron is observed in anaemic patients thus helping in classification of iron disturbance and monitoring the therapy.
Causes of anaemia include a number of factors and classification of anaemia is an important step in determining the cause (Aird, 2000; Morrison, 2005).
The present study is directed to observe the type of iron metabolism disturbance occurring in anaemic dogs with hemoprotozoal parasite infection as limited data is available regarding iron metabolism disturbance in dogs with hemoprotozoal infection.
The present study was carried out in dogs of any age group and breed affected with hemoprozoal infection with clinical signs suggestive of anaemia with alteration in complete blood count (CBC) profile value. Clinical examination of the dogs was performed as described by Ettinger and Feldman (2005). The serum iron profile analytes (serum iron, serum TIBC, %TSAT) was also measured. The study included 6 apparently healthy dogs as control groups which had a proper history of vaccination, deworming and were negative for any presence of diseases.
Out of 1247 cases examined at Department of TVCC Bombay Veterinary College, Parel, 20 clinically anaemic cases showed haemoglobin less than 10g% and PCV less than 37 percent. The overall prevalence of anaemia was 1.60%. Out of which 6 cases showed positive for hemoprotozoal infection.
Blood was collected in sterile vacuttainers (LS ECOTAINER EDTA K3). Serum was separated by using the LS ECOTAINER Plain vacuttainer tubes. Hematological parameters under study that included hemoglobin (Hb), total erythrocyte count (TEC), packed cell volume (PCV), reticulocyte count, platelet count, white blood corpuscular count (WBC), differential leucocyte count (DLC), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) was evaluated by the method as described by Benjamin (2007). Serum biochemistry parameters under study that included Alkaline phosphatase(ALP), serum glutamate pyruvate transaminase(SGPT), serum glutamic-oxaloacetic transaminase(SGOT), total serum protein, albumin, globulin, Albumin/Globulin ratio (A/G ratio), Blood Urea Nitrogen (BUN), urea, creatinine, total bilirubin (TB), direct bilirubin (DB) and indirect bilirubin (IB) were estimated by spectrophotometry in robonik prietest touch auto biochemistry analyser using the supplied reagent (M/s Span diagnostics, Kolkata). SGPT and SGOT were dertermined by IFFC method (Reitman and Frankel, 1957), total protein by biuret method (Gornall et al., 1949), albumin by BCG method (Gustaffson, 1978), total and direct bilirubin by diazo method (Ertingshausen et al., 1973), urea by GLDH-urease method (Tietz, 1995) and creatinine by Jaffe’s reaction (Vasiliades, 1976). Indirect bilirubin was calculated as [IB= TB- DB]. Albumin/Globulin ratio (A/G ratio) was calculated as [A/G ratio= Albumin/Globulin] Serum iron profile study parameters that such as serum iron, serum total iron binding capacity (TIBC) and percent transferrin saturation (%TSAT) were estimated by I-lab 600 acute auto analyser using the supplied reagent (Sigma Diagnostics, St. Louis, (USA). Serum iron was determined by Ferene method (Smith et al. 1981), serum TIBC by biochemical method (Henery, 1984). Transferrin saturation was calculated by the following formula:
% TSAT= (Serum iron/ TIBC) x 100
Cases with Ehrlichia canis infection were confirmed by using SNAP 3Dx kit (M/s IDEXX Laboratories, New Delhi) that detects Ehrlichia canis antibodies. Blood smear for hemoprotozoal parasite identification was prepared on a clean and dry glass slide with a drop of blood collected from the ear tip and stained with Leishman’s stain.
Normally distributed data are reported as mean ± standard error. Student’s t-test was used for statistical analysis. Statistical significance was set at P≤ 0 .05. This was performed by using the analysis tool pack software of Microsoft Excel 2007.
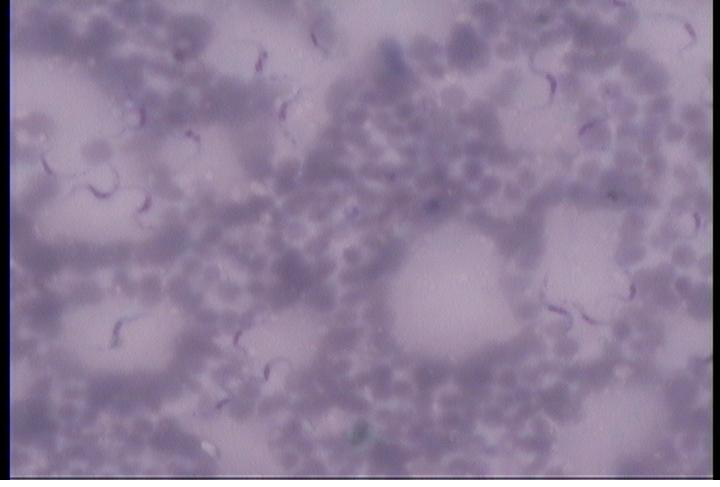
Out of the 6 cases positive for hemoprotozoal infection, it included 4 cases of Ehrlichiosis detected by SNAP 3Dx kit (Figure 1) and 2 cases of trypanosomiasis (Figure 2). Prominent clinical signs observed was fever, anorexia, pale mucous membrane, lymphadenopathy, spleenomegaly and epistaxis. The red blood cell cellular morphology showed hypochromasia and microcytosis.
The hemato-biochemical alterations when compared to the apparently healthy control dogs (Table 1) showed statistically significant difference regarding parameters Hb, TEC, reticulocyte count and platelet count. Statistical significant difference was also observed in the TP, albumin, A/G ratio and BUN parameters when compared with the apparently healthy control group. Apart from the above, the cases also showed leucopenia with neutropenia and lymphopenia, hypoalbuminemia, hyperglobulinemia, hyperbilirubinemia, increase in ALT, AST and ALP levels.
Table 1: Mean±SE values of CBC, LFT, KFT in apparently healthy and anaemic dogs
|
Parameters |
Control group |
Clinical cases |
|
Hb (g %) |
13.38 ± 0.37 |
7.10 ± 1.09* |
|
T.E.C(×106/µl) |
5.76 ± 0.13 |
2.92 ± 0.45* |
|
P.C.V (%) |
40.15 ± 1.12 |
22.4 ± 3.01* |
|
Reticulocyte count (%) |
1.67 ± 0.33 |
1.17 ± 0.17* |
|
W.B.C(×103/µl) |
11450 ± 1393.26 |
10250 ± 1061.68 |
|
Platelets(×105/µl) |
283666.70 ± 25935.39 |
132000 ± 17970.3* |
|
M.C.V (fL) |
69.67 ± 1.09 |
79.92 ± 4.33 |
|
M.C.H (pg) |
23.18 ± 0.37 |
24.77 ± 1.36 |
|
M.C.H.C (g/dl) |
33.25 ± 0.07 |
31.12 ± 1.24 |
|
T.B(mg/dl) |
0.32 ± 0.048 |
0.80 ± 0.11 |
|
D.B(mg/dl) |
0.17 ± 0.03 |
0.40 ± 0.10 |
|
I.B(mg/dl) |
0.15 ± 0.03 |
0.40 ± 0.06 |
|
S.G.P.T(IU/L) |
81.33 ± 3.13 |
101.885 ± 60.316 |
|
S.G.O.T(IU/L) |
53.52 ± 2.89 |
137.98 ± 60.32 |
|
A.L.P(IU/L) |
94.87 ± 3.65 |
179.6 ± 71.07 |
|
T.P(g/dl) |
8.10 ± 0.15 |
6.91 ± 0.46* |
|
Albumin(g/dl) |
3.90 ± 0.20 |
1.89 ± 0.31* |
|
Globulin(g/dl) |
4.37 ± 0.16 |
5.01 ± 0.32 |
|
A/G |
0.89 ± 0.06 |
0.37 ± 0.16* |
|
BUN(mg/dl) |
8.99 ± 0.35 |
22.25 ± 5.95* |
|
Urea(mg/dl) |
19.21 ± 0.761 |
47.68 ± 12.73 |
|
Creatinine(mg/dl) |
0.84 ± 0.07 |
1.26 ± 0.13 |
*Significant (P ≤ 0.05) at t-crit (2.06)
Table 2: Mean ±SE value of serum iron profile of apparently healthy and anaemic dogs
|
Parameters |
Control group |
Clinical cases |
|
Serum iron (µg/dl) |
141.83 ± 7.91 |
109.32 ± 22.96* |
|
Serum TIBC (µg/dl) |
319.33 ± 31.89 |
239.3 ± 34.99 NS |
|
TSAT (%) |
47.53 ± 6.54 |
50.47 ± 9.79 NS |
*Significant (P ≤ 0.05) at t-crit (2.06); NS- Non Significant
Table 3: Mean ± SE values of CBC in clinical cases before and after treatment
|
Parameters |
Before treatment |
After Treatment |
t-stat |
t-crit |
|
Hb (g %) |
7.10 ± 1.09 |
9.15 ± 1.04 |
4.77* |
2.57 |
|
T.E.C (×106/µl) |
2.92 ± 0.45 |
4.25 ± 0.29 |
5.81* |
|
|
PCV (%) |
22.40 ± 3.01 |
28.92 ± 3.53 |
4.07* |
|
|
WBC (×103/µl) |
10250 ± 1061.68 |
12400 ± 3023.91 |
0.83 |
|
|
Reticulocyte count (%) |
1.17 ± 0.17 |
2.17 ± 0.28 |
4.47* |
|
|
Platelets (×105/µl) |
132000 ± 17970.35 |
185333.3 ± 18895.62 |
3.69* |
|
|
MCV(fL) |
79.92 ± 4.33 |
67.37 ± 6.14 |
1.70 |
|
|
MCH(pg) |
24.77 ± 1.36 |
20.86 ± 1.42 |
4.64* |
|
|
MCHC(g/dl) |
31.12 ± 1.24 |
31.81 ± 0.71 |
0.38 |
* Significant (P ≤ 0.05)
Table 4: Mean ± SE values of LFT in clinical cases before and after treatment
|
Parameters |
Before treatment |
After treatment |
t-stat |
t-crit |
|
T.B.(mg/dl) |
0.80 ± 0.11 |
0.45 ± 0.03 |
2.99* |
2.57 |
|
D.B.(mg/dl) |
0.40 ± 0.10 |
0.2 ± 0.05 |
1.88 |
|
|
I.B.(mg/dl) |
0.40 ± 0.06 |
0.23 ± 0.03 |
3.96* |
|
|
SGPT(IU/L) |
101.89 ± 25.91 |
72.17 ± 6.14 |
1.31 |
|
|
SGOT(IU/L) |
137.98 ± 60.32 |
102.59 ± 16.79 |
0.78 |
|
|
ALP(IU/L) |
179.6 ± 71.07 |
209.27 ± 29.59 |
0.60 |
|
|
TP(g/dl) |
6.91 ± 0.46 |
7.10 ± 0.53 |
0.65 |
|
|
Albumin(g/dl) |
1.89 ± 0.31 |
2.37 ± 0.27 |
4.25* |
|
|
Globulin(g/dl) |
5.01 ± 0.32 |
4.57 ± 0.28 |
1.82 |
|
|
A/G ratio |
0.37 ± 0.07 |
0.52 ± 0.06 |
5.13* |
*Significant (P ≤ 0.05)
Table 2 depicts the serum iron profile between the control group and clinical cases and it showed statistical significant difference between the serum iron parameter and non-significant difference between serum TIBC and %TSAT. There was also a decrease in serum TIBC levels compared to the control group.
The affected cases were therapeutically managed as per the standard line of treatment. Cases with E. canis were treated with Doxycycline @ 5mg/kg B.W. for 15 days and cases with Trypanosoma sp. evansi infection were treated with Triquin (Quinapyramine Sulphate and chloride) at 0.025ml/kg B.W. once. No adverse outcomes were observed from the cases during the therapy. The hemato-biochemical and serum iron profile parameters were re-evaluated in the affected cases after clinically recovery and is summarized in Table 3, 4, 5 and 6. Statistical significant difference was observed in Hb, TEC, PCV, reticulocyte count, platelet count, MCH, TB, IB, Albumin, A/G ratio, BUN, urea and creatinine parameters after therapeutic intervention. Among the serum iron profile, statistical significant difference was observed in serum iron parameters.
The clinical findings were in accordance as observed by Harrus et al. (1997), Harrus et al. (1998) and Warner and Harrus (2000). The results showed alterations in the hemato-biochemical and serum iron profile parameters when compared to the control group suggesting the presence of anemia with disturbance in the iron metabolism.
Giger (2005) came up with the opinion that low values of Hb, TEC and PCV in the cases may be attributed to deficiency of iron, destruction of red blood corpuscles and/or excess blood loss and reduced erythropoietic activity. Waner (2008) also stated that anaemia in Ehrlichia sp. infection is attributed to direct effect of the organism on the red blood cells. Aquino et al. (2002) and Abenga et al. (2005) also reported low PCV, Hb, and RBC in Trypanosoma infections. There was also leucopenia, neutropenia and lymphopenia in the affected animals which coincided with the findings of Warner and Harrus (2000). The alterations in the T.P., Albumin, A/G ratio was reported by Supriya et al. (2011) in clinical case with ehrlichiosis. There was also alteration in AST, ALT, ALP and hyperbilirubinemia and it was either due to secondary to hyperglobulinemia as a compensatory mechanism for the maintenance of normal blood viscosity increased by high globulin levels or due to increase in the immunoglobulin levels as a result of the hemoprotozoal infection (Aquino et al., 2001).
Table 5: Mean ± SE values of KFT in clinical cases before and after treatment
|
Parameters |
Before Treatment |
After Treatment |
t-stat |
t-crit |
|
BUN (mg/dl) |
22.25 ± 5.95 |
10.56 ± 1.76 |
2.72* |
2.57 |
|
Creatinine (mg/dl) |
1.26 ± 0.13 |
0.89 ± 0.10 |
2.74* |
|
|
Urea (mg/dl) |
47.68 ± 12.73 |
22.62 ± 3.77 |
2.72* |
*Significant (P ≤ 0.05)
Table 6: Mean ± SE values of serum iron profile in anemic cases before and after treatment
|
Parameters |
Before treatment |
After treatment |
t-stat |
t- crit |
|
S. iron (µg/dl) |
109.32 ± 22.96 |
124.18 ± 25.47 |
3.33* |
2.57 |
|
S. TIBC (µg/dl) |
293.30 ± 34.99 |
253.20 ± 31.54 |
1.78 |
|
|
TSAT (%) |
50.47 ± 9.79 |
52.69 ± 9.792 |
1.44 |
*Significant (P ≤ 0.05)
Serum iron and TIBC were lower than the control group, suggesting that iron metabolism disturbance does occur in hemoprotozoal infection and the disturbance is of the order seen in anemia due to inflammatory disease (Lillihook et al., 1998 and Furnanello et al., 2005). These lower levels are due to iron retention within macrophages resulting from increased uptake of iron by different pathways as well as inhibition of iron recycling by blockage of iron export (Stijlemans et al., 2008) and can be accredited as defence mechanism of body against infection to withhold iron utilization by the invading microbes for their growth (Fry, 2011 and McCown and Specht, 2011). Kelly et al. (2013) observed microcytic anaemia as the cellular changes in dogs affected with ehrlichiosis. Silva et al. (1995) and Gunaseelan et al. (2009) reported the presence of microcytic hypochromic cellular changes in dogs affected with Trypanosoma evansi. The prominent cellular changes observed in our study showed microcytosis and hypochromasia and is in accordance with the opinion of the above mentioned authors. The reason for the microcytosis in some dogs has been proposed to be associated with chronic inflammatory disease as a result of prolonged sequestration of storage iron and microcytosis has been reported in many different types of inflammatory disease in dogs, including gastrointestinal, infectious, respiratory and skin disease (Gavazza et al., 2012).
Use of Doxycycline and Triquin (quinapyramine sulphate and chloride) for ehrlichiosis and trypanosomiosis, respectively, for therapy was followed by Mylonakis et al. (2011) and Juyal (2011). The significant changes on the post treatment levels on the parameters can be attributed due to the etiological specific therapy this also resulted in restoration of levels of serum iron analytes to normal without any exogenous use of iron supplementation.
In conclusion the results from this study, hemoprotozoal diseases will result in disturbance of iron metabolism subsequently leading to anaemia. The characteristics of the iron metabolism disturbance in present study were analogous to anaemia of inflammatory diseases. From the above iron disturbances observed in the study it can also be inferred that therapy should not involve exogenous iron administration since it may lead to aggravation of the existing infection through microbial utilization of iron. Hence serum iron profile studies indeed are a valuable aid in the diagnosis of iron metabolism disturbances and should be included as a routine diagnostic parameter in the anaemic dogs.
CONFLICT OF INTEREST
There is no conflict of interest among all or any of the authors.
ACKNOWLEDGEMENT
We thank the Associate Dean, Bombay Veterinary College for providing the necessary facility required for the study.
REFERENCE