Advances in Animal and Veterinary Sciences
Research Article
Evaluation of Nuclear Factor Kappa Beta, Nitric Oxide and Blood Neutrophil/Lymphocyte Ratio as Biomarkers of Inflammatory Response and Complementary Therapy in Dogs with Experimental Skin Pseudomonas aeruginosa Infection
Maria Andonova1*, Valentina Urumova2, Dimitrichka Dimitrova3, Evgeni Slavov1, Petko Dzhelebov1, Tsvetan Chaprazov4, Ivan Borissov4
1Department of General and Clinical Pathology; 2Department of Veterinary Microbiology, Infectious and Parasitic Diseases; 3Department of Pharmacology, Animal Physiology and Physiological Chemistry; 4Department of Veterinary Surgery, Faculty of Veterinary Medicine, Trakia University, Stara Zagora 6000, Bulgaria.
Abstract | The optimisation of strategies for control of Pseudomonas aeruginosa skin infections in dogs includes minimization of induced hyper-inflammation. We aimed at monitoring changes in serum nuclear factor kappa B (NF-кB) and nitric oxide (NO) concentrations, and blood neutrophil/lymphocyte ratio (N/L ratio), to evaluate their potential as inflammatory markers throughout the course of therapy in canine skin infection experimentally induced by P. aeruginosa. A complementary therapy including antibiotic against the pathogen and NF-кB targeted host modulatory therapy by parthenolide from the phytopreparation Feverfew was applied. NF-кB was determined by ELISA kit. The NO assay was based on the griess reaction. The N/L ratio was calculated on the basis of CBC counts. The infection was induced by P. aeruginosa (1×108 CFU/mL) injected s.c. to 20 male dogs. Four experimental groups were formed (0 – untreated dogs and three treated groups: I – with antibiotic; II – with Feverfew and III – with antibiotic and Feverfew) and one control group. The results showed that NF-кB in group 0 was significantly higher than those in controls on hour 24 (p=0.0103), hour 48 (p=0.0001), hour 72 (p=0.0505), day 7 (p=0.0114), and day 10 (p=0.0094). The treatment with Feverfew (group II and III) achieved an efficient control on NF-кB. In groups I and II, biphasic increase in NO was observed, associated with the early – hour 4 (p=0.00007 for group I; p=0.0001 for group II vs. baseline) and late – day 14 (p=0.0092 for group II vs. controls) stage of infection development. By hour 24, N/L ratio in these groups increased (p=0.005 and p=0.0104 for groups I and II respectively vs. baseline). The established changes were generalised signs of inflammatory response accompanied by fever on hour 4 in infected dogs (p=0.0005 for group 0; p=0.0008 - I; p=0.0002–II; p=0.0047-III vs. controls).
Keywords | Pseudomonas aeruginosa, Canine skin infection, Inflammatory biomarkers, Therapy, Feverfew
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | January 20, 2015; Revised | February 12, 2015; Accepted | February 13, 2015; Published | February 20, 2015
*Correspondence | Maria Andonova, Trakia University, Stara Zagora, Bulgaria; Email: [email protected]
Citation | Andonova M, Urumova V, Dimitrova D, Slavov E, Dzhelebov P, Chaprazov T, Borissov I (2015). Evaluation of nuclear factor kappa beta, nitric oxide and blood neutrophil/lymphocyte ratio as biomarkers of inflammatory response and complementary therapy in dogs with experimental skin Pseudomonas aeruginosa infection . Adv. Anim. Vet. Sci. 3(3): 174-182.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.3.174.182
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Andonova et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Pseudomonas aeruginosa is an opportunistic pathogen, which attacks the body after breakthrough of skin immune protection (Kipnis et al., 2006). It is a skin commensal in dogs and causes skin infections that are difficult to control. Antibiotic therapy is problematic, due to the high bacterial variability, biofilm forming and swift building of resistance to antibiotics (Breidenstein et al., 2011). The challenge with P. aeruginosa infections is also due to the heterogenous nature of occurring disturbances affecting systemic defense - innate immunity (Lavoie et al., 2011) and adaptive immunity (Sprucek et al., 2007). P. aeruginosa induces a strong inflammatory response (Kumar et al., 2011) through activation of many skin cell elements, enhanced synthesis of pro-inflammatory cytrokines, strong neutrophil infiltration (Mantovani et al., 2011). The inflammation process requires great amount of metabolic energy and provokes a systemic response manifested with fever, metabolic and functional alterations (Lindestam, 2010). In a clinical setting, the thorough control on inflammatory response strength is very important because the uncontrolled initial inflammation may overshoot and result in tissue damage and immune dysfunction. This necessitates to identify biomarkers, which could assess the development of inflammation, with predictive power and offering possibilities for evaluation of applied treatment. Some of them are haematological and blood biochemical indices (Andonova et al., 2014), cytokines (Lacour et al., 2001), although observed changes are only short-term. Furthermore, the search for effective therapy has recently become most intensive (Angus, 2011). The optimisation of strategies for eradication of P. aeruginosa requires a complementary therapy including antibiotic against the pathogen and host modulatory therapy to correct impaired key signalling pathways. NF-кB is a target of host modulatory therapy (Vitiello et al., 2012). NF-кB controls genes, a major part of which are related to inflammatory process (Tilstra et al., 2011). Tak and Firestein (2001) and Rahman and McFadden (2011) consider NF-kB as a biomarker of inflammation. Xie et al. (1994) comment on the role of NF-kB in induction of nitric oxide (NO) synthesis. Yang et al. (2013), Celikbilek et al. (2014) and Farah and Khamisy-Farah (2014) outlined the neutrophil/lymphocyte ratio (N/L ratio) is a simple marker of inflammation. Many efforts have been focused on identifying substances, which can prevent the inflammatory process at the very early stage of gene expression. Paur et al. (2008) reported that plant extracts are efficient modulators of NF-кB. Several groups have reported the effects of parthenolide (natural product, a sesquiterpene lactone isolated from extracts of Tanacetum parthenium - Feverfew) on NF-кB inhibition. Koprowska and Czyz (2010) outlined the molecular mechanisms of parthenolide action, whereas Mathema et al. (2012) are focused on anti-inflammatory properties of this natural product.
The multi-pharmacological potential of parthenolide, the limited clinical data about dosage, onset and duration of application and its efficacy as anti-inflammatory agent in dogs, the possibility for utilisation of easy-to-detect inflammation biomarkers in a clinical setting, and tests of new therapeutic schedules in skin P. aeroginosa infection based on the pathogenetic approach have provoked our scientific interest.
We designed and performed an in vivo study in dogs with experimental P. aeruginosa model of skin infection aimed at monitoring changes in serum NF-кB and NO concentrations, and blood N/L ratios, to evaluate their potential as inflammatory markers throughout the course of agent- or/and host-targeted therapy for infection control.
MATERIALS AND METHODS
Animals
Twenty-five clinically healthy adult mixed-breed male dogs from a licensed vivarium, 2 to 5 years of age were used. Prior to the experiment they were treated with antiparasitic shampoo Friskies (Milano–Italia), orally with praziquantel/abamectin (Prazimec D–Biovet Ltd, Peshtera, Bulgaria) against helminths at a dose of 1 tablet/10 kg body weight and with the combination permethrin/carbaryl against ectoparasites (Tapilan-B − Dorvert, Israel). The dogs were housed in the vivarium of the Functional Pathology and Immunology Unit at the Faculty of Veterinary Medicine under the following conditions: mixed light regimen (light/dark), air humidity 50-60% and air temperature 15-21oC. They were fed dry canine food (Canil Social Gouomarc H − Brazil) and had a free access to drinking water. Dogs were walked twice daily.
Experiments were performed with strict compliance with animal ethics standards (protocol 16/2010 of the Ethical Committee of National Veterinary Service; permit No. 37). All dogs included in the trial were treated appropriately; the clinical and blood laboratory indices were regularly analysed. The health and microbiological status of dogs were strictly monitored. The dogs received the best possible veterinary care from experienced personnel. After the end of the trial, they did not pose any risk for the environment, public health and their owners.
Bacterial Strain
A canine field P. aeruginosa isolate typed by the BBL Crystal semi-automated identification system (Becton Diskinson, USA) and kit for Gram-negative bacteria and non-fermentative bacteria was used. The kit contains thirty enzymatic tests for biochemical identification of bacteria. The following characteristics of P. aeruginosa were used for presumptive identification: growth on MacConkey agar; pyoverdin production; oxidase test; growth at 5oC and at 42oC; oxidation of glucose, lactose, maltose; arginine dehydrolase production; urease production; reduction of nitrate to nitrite; urease production; gelatin hydrolisation.
Experimental Design
Experimental skin infection model was performed through subcutaneous injection of P. aeruginosa suspension in sterile saline into a depilated area in the neck region of dogs (n=20). We used a bacterial culture in stationary phase with density corresponding to 1×108 CFU/mL prepared nephelometrically according to the MacFarland standard (Quinn et al., 1999). Infected animals were further divided into groups. Those from group 0 (n=5) were injected only with P. aeruginosa. Dogs from group I (n=5) were treated s.c. with 2.5% Enrofloxacin solution (Syvaquinol 25 injectable − Syva laboratorios, Spain) on post infection hour 48. The antibiotic therapy lasted 10 consecutive days at a dose of 5 mg/kg. Dogs from group II (n=5) were treated with Feverfew (standardised extract, active principle parthenolide 0.7% − Nature’s Way, USA), by application of 1 capsule at 12-hour intervals. The treatment per os, began on post infection hour 4 and continued for 6 days. The purpose of applying this natural product in the early stage of skin infection was to control the inflammation response strength (host modulatory therapy). Dogs from group III (n=5) were treated with the combination of antibiotic and Feverfew. The schedule consisted of daily oral intake of two capsules Feverfew for 6 days and the s.c. antibiotic therapy with Enrofloxacin started on the 48th hour post infection and lasted 10 days. The control group (n=5) was s. c. injected with saline into a depilated area of the neck.
Collection of Blood Samples
Blood samples were obtained from v. cephalica antebrachii by means of Venflon cannulae (Vygon GmbH & Co., Germany) prior to the infection – hour 0 and on post infection hours 4, 24, 48, 72 and days 7, 10 and 14. All blood samples were collected in the morning before feeding (8.00−8.30 AM) to eliminate circadian influences in EDTA-anticoagulated and serum separator tubes. Samples were allowed to clot for two hours at room temperature before centrifugation for 15 min at 1000×g. Sera were removed, assayed immediately (for determination of serum NO concentrations) or aliquoted and stored at −80oC (for determination of NF- кB).
Serum concentrations of canine nuclear factor kappa B (NF-кB) were determined by a commercial ELISA test kit (cat. No E1824c, USCN LIFE, China). This assay recognises recombinant and natural canine NF-кB without significant cross-reactivity or interference. The detection range was from 0.312 to 20 ng/ml.
Serum NO was measured as stable end product nitrite according to the method of Miranda et al. (2001). Serum samples were deproteinised with cold acetonitrile (1:1). After centrifugation (10 min; 1000×g), supernatants were analysed immediately. The assay is based on the reduction of nitrate by vanadium trichloride combined with detection by the acidic griess reaction. The diazotisation of sulfanilic acid with nitrite at acidic pH and subsequent coupling with N-(10 naphthyl)-ethylene diamine produced a coloured compound measured spectrophotometrically at 540 nm. The results are presented in µmol/L.
The blood neutrophil to lymphocyte (N/L) ratio was calculated on the basis of CBC counts obtained from the automated cell count analyzer Coulter Electronics, Krefeld, German.
Statistical Analysis
Results are presented as mean ± SD. Data was submitted to one-way ANOVA followed by Turkey’s post hoc test by means of statistical software (Graph Pad InStat3; MedCalc, Belgium). Differences were considered statistically significant at the p<0.05 level.
RESULTS
The dynamics of NF-кB concentrations depicted in Figure 1 shows similar levels in all experimental dogs on post infection hour 4. However, on post infection hour 24, the animals from group 0 and group I exhibited peaks in NF-кB concentrations, which were statistically significant both vs hour 4 (p=0.0123 and p=0.0128 respectively), and vs controls, groups II and III. Dogs from group 0 maintained significantly higher NF-кB levels even on post infection day 10 compared to controls (p=0.0094), group II (p=0.0265) and group III (p=0.0097). Dogs treated with Feverfew (group II) and Enrofloxacin + Feverfew (group III) did not show any considerable deviations in serum NF-кB throughout the experiment (Figure 1). This was also observed in dogs from group I (antibiotic therapy), whose NF-кB levels were not substantially altered between post infection days 7 and 10.
In all infected animals, serum NO increased on post infection hour 4 as compared to baseline, with statistically significant differences in groups I (p=0.00007) and II (p=0.0001) (Table 1). In group III, NO levels were higher both vs controls (p=0.0386). After that time, serum NO concentrations in the group treated with antibiotic declined significantly by post infection hours 24 (p=0.0060); 48 and 72 (p=0.0001) and day 10 (p=0.0064) vs hour 4. Dogs treated with Feverfew (group II) also exhibited a substantial reduction, with minimum value on the 7th day. In these two groups however, NO concentrations increased again at the end of the experiment (day 14) with statistically significant differences between group II vs controls (p=0.0092) and vs group 0 (p=0.0040). At that time, NO levels in other infected dogs (groups 0 and III) were also higher than controls.
Four hours after s. c. inoculation of P. aeruginosa, N/L ratios in all experimental animals were slightly higher than baseline values (Table 2). In dogs treated with Feverfew (group II), N/L ratios attained a peak on post infection hour 24, which was considerably difference both vs control group (p=0.0082), and group 0 (p=0.028). After that period, N/L ratio in group II were significantly reduced on day 7 (p=0.0358), 10 (p=0.0086) and 14 (p=0.0120) vs hour 24. A similar time course was observed in infected dogs treated with Enrofloxacin (group I) except for the lower peak at hour 24 and the minimum N/L ratio occurring at the 14th day (p=0.0002 vs hour 24; Table 2).
On post infection hour 4, rectal temperature (RT) was increased in all experimental groups compared to control (p=0.0005 for group 0, p=0.0008 for group I, p=0.0002 for group II and p=0.0047 for group III) (Table 3). On hour 48, the fever was still present in all groups except for group III. Another 24 h later, the RT of dogs in group 0 only remained elevated compared to both controls (p=0.0136) and dogs treated with Feverfew+enrofloxacin (p=0.0076). On subsequent experimental time intervals, RT in all groups was within the normal range.
The described changes in dogs with experimental skin P. aeruginosa infection were accompanied by nonspecific symptoms and signs – inappetance, somnolence associated with fever and were related to the organ involved. At the site of injection, inflammation was detected as early as the 4th hour and on hour 24, round firm non-painful infiltrative oedema, with erythematous plaques have appeared. The area became indurated, and the skin – rough and crusty (days 7–10).
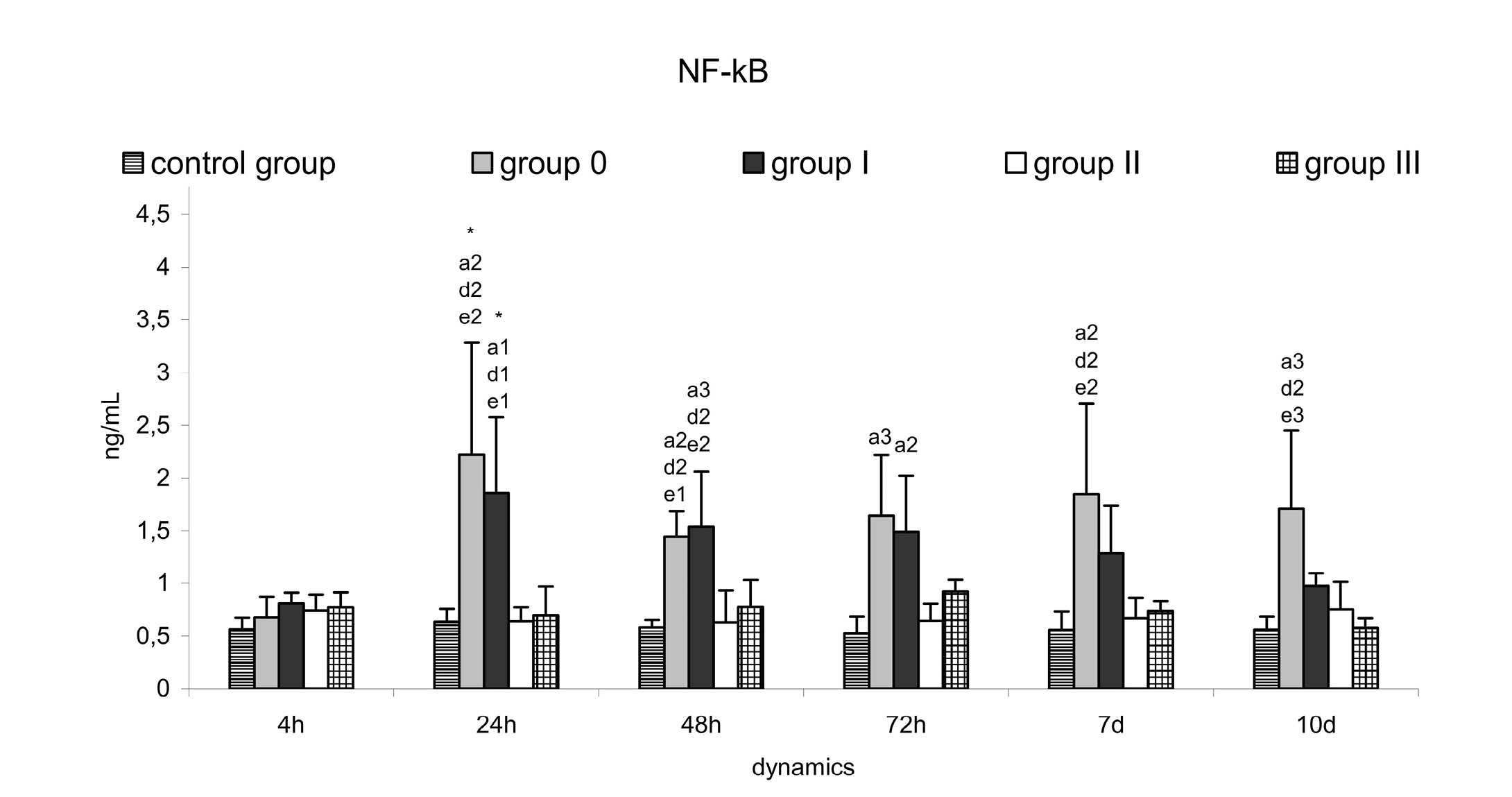
Figure 1: Serum nuclear factor kappa-beta (NF-kB) (ng/mL) in controls, dogs with experimental skin P. aeruginosa infection - group 0 (untreated) and treated: group I (antibiotic), group II (Feverfew); group III (antibiotic+Feverfew)
Values are means ± SD of five dogs in each group.
Between-group level of significance: 1 p<0.05; 2 p<0.01; 3 p<0.001; a – vs control group; d – vs group II; e – vs group III.
Table 1: Serum nitric oxide (μmol/L) in controls, dogs with experimental skin P. aeruginosa infection - group 0 (untreated) and treated: group I (antibiotic), group II (Feverfew); group III (antibiotic+Feverfew). Values are means ± SD of five dogs in each group
|
Groups |
|||||
|
Control |
0 group |
I group |
II group |
III group |
|
|
0h |
66.6±2.7 |
64.4±10.9 |
56.9±7.8 *** |
64.8±4.8 * |
64.4±5.0 |
|
4 h |
65.4±2.3 |
71.2±14.8 |
92.2±0.5 +++ |
95.8±8.8 |
110.8±41.0 a1 |
|
24h |
65.4±4.8 |
69.8±9.7 |
58.4±20.4 ++ |
70.2±20.1 |
88.4±21.4 |
|
48 h |
64.8±4.7 |
70.6±8.1 |
62.5±9.0 +++ |
68.0±5.2 |
72.5±21.4 |
|
72 h |
66.3±3.9 |
70.5±15.0 |
59.8±10.6 |
81.8±9.2 ++ |
84.7±41.9 |
|
7 d |
64.9±5.0 |
101.4±46.2 |
76.9±10.4 ++ |
55.0±5.5 |
77.4±13.6 |
|
10 d |
62.8±4.0 |
81.9±6.9 |
66.5±15.7 ***§§vv |
85.4±7.8 ***§§vv••• |
85.6±21.1 |
|
14 d |
63.9±4.6 |
73.6±14.5 |
87.9±4.8 |
109.9±29.8 a2b1 |
85.3±20.2 |
Within-group level of significance: * p<0.05; **p<0.01; ***p<0.001 vs 0 h; ++р<0.01; +++p<0.001 vs 4 h; §§ p <0.01 vs 24 h; vv р<0.01 vs 48 h; ••• p<0.001 vs 7 d
Between-group level of significance: 1 p<0.05; 2 p<0.01; а – vs control group; b – vs group 0
Table 2: Blood neutrophil/lymphocyte ratio in controls, dogs with experimental skin P. aeruginosa infection - group 0 (untreated) and treated: group I (antibiotic), group II (Feverfew); group III (antibiotic+Feverfew). Values are means ± SD of five dogs in each group
|
Groups |
|||||
|
Control |
0 group |
I group |
II group |
III group |
|
|
0h |
2.04±0.34 |
2.53±1.32 |
2.63±0.43 |
2.67±1.65 |
1.72±0.91 |
|
4 h |
2.59±0.18 |
3.38±1.35 |
5.19±1.80 |
5.26±1.04 |
5.03±2.52 |
|
24h |
2.53±0.72 |
3.88±2.81 |
7.90±1.98 ** |
11.44±5.66 **a2b1 |
5.28±2.63 |
|
48 h |
1.92±0.21 |
5.21±3.84 |
6.30±2.72 |
7.17±2.68 |
4.72±2.56 |
|
72 h |
2.36±0.30 |
5.08±2.64 |
6.23±2.88 |
5.76±4.09 |
3.51±1.35 |
|
7 d |
2.66±0.94 |
3.46±1.70 |
4.05±1.70 |
4.34±2.77 § |
2.14 ± 1.34 |
|
10 d |
2.33±0.17 |
2.85±1.23 |
2.88±0.90 |
2.64±0.58 §§ |
2.70±1.53 |
|
14 d |
2.58±0.46 |
1.88±0.76 |
1.78±0.62 §§§v |
3.10±1.11 §§ |
2.78± 2.49 |
Within-group level of significance: * p<0.05; **p<0.01; ***p<0.001 vs 0 h; § p<0.05; §§ p <0.01; §§§ p<0.001 vs 24 h; v p<0.05 vs 48 h
Between-group level of significance: 1 p<0.05; 2 p<0.01; а – vs control group; b – vs group 0
DISCUSSION
The opportunistic Gram-negative bacterium P. aeruginosa is a major pathogen, causing both local and generalised infections (Van Delden, 2007). The proper functioning of natural skin defense mechanisms – barrier systems, cellular and humoral elements, non-specific responses – inflammation, acute phase response, phagocytosis, guarantee the discontinuation of infection during the acute phase, when adaptive immunity is not yet realized (Meglio et al., 2011). The pathogenesis of Pseudomonas aeruginosa appears to be complex and multifactorial, impeding the application of the pathogenetic therapeutic approach. The selection of a specific therapeutic strategy was aimed at restoration of the equilibrium in defense systems through elimination of unwanted responses. Our experimental model of canine skin P. aeruginosa infection allowed to monitor in vivo not only the systemic defense mechanisms during the development of infection (from the 4th hour to the 14th day) but also to evaluate occurring injuries as well as the efficacy of applied treatment. All dogs infected with P. aeruginosa developed inflammation at the site of inoculation with typical swelling, reddening and induration of the area. The evaluation of inflammation strength is important as the extremely intense inflammation could provoke severe tissue damage and systemic effects - fever, induced by pro-inflammatory cytokines, metabolic and functional changes (Lindestam, 2010), as well as haematological changes (Andonova et al., 2014). From this point of view, we sought some sensible biomarkers of inflammation, reacting even in patients with slight inflammatory response to the inoculated agent. The number of studies on inflammation biomarkers in the scientific literature is remarkable (Lacour et al., 2001). The advantages of inflammation biomarkers studied in this experiment were availability of specimens and rapid assay, presence both in the acute and chronic stage of inflammation, possibility for reduction of the number of ordered clinical tests with respect to lowering costs without compromising the quality of information.
Table 3: Rectal temperatute (C) in controls, dogs with experimental skin P. aeruginosa infection - group 0 (untreated) and treated: group I (antibiotic), group II (Feverfew); group III (antibiotic+Feverfew). Values are means ± SD of five dogs in each group
|
Groups |
|||||
|
Control |
0 group |
I group |
II group |
III group |
|
|
0h |
38.8±0.24 |
38.6±0.62 |
38.2±0.38 |
38.6±0.22 |
38.8±0.32 |
|
4 h |
38.7±0.20 |
40.0±0.48 a3*** |
39.7±0. 38 a2*** |
40.0±0.40 a3** |
39.7±0.54 a1* |
|
24h |
38.7±0.13 |
40.3±0.37 *** |
39.4±0.63 ** |
40.2±0.58 *** |
39.8±0.24 * |
|
48 h |
38.6±0.28 |
40.3±0.36 *** |
39.4±0.58 ** |
39.2±0.47 ** |
39.6±0.38 |
|
72 h |
38.7±0.50 |
39.5±15.0 a1e1*§v |
39.3±0.24 ** |
39.4±0.50 |
38.8±0.35 |
|
7 d |
38.7±0.46 |
38.8±0.27 |
38.8±0.38 |
38.6±0.70 |
38.6±0.50 |
Within-group level of significance: * p<0.05; **p<0.01; ***p<0.001 vs 0 h; § p<0.05 vs 24 h; v p<0.05 vs 48 h
Between-group level of significance: 1 p<0.05; 2 p<0.01; а – vs control group; e – vs group III
In their analysis of inflammatory response to infection and having reviewed the molecular biology of inflammation and sepsis, Cinel and Opal (2009) emphasised the key position of NF-кB. NF-кB is one of the most important transcription factors with a potential for regulation of a number of biological processes – inflammation, acute phase response, immune response (Hayden et al., 2006). Cigana et al. (2011) outlined that via NF-кB activation, P. aeruginosa stimulates proinflammatory cytokine production. Our in vivo experiments with P. aeruginosa demonstrated 2-3 times higher serum NF-кB concentrations as early as the 24th hour after inoculation in dogs from groups 0 and I (Figure 1). This confirms that NF-кB could be a reliable marker in skin infections, which is also seen from the fact that its levels persisted high even by the 10th day in untreated dogs. The key signalling pathway triggered by P. aeruginosa could be hardly distinguished in in vivo studies, as the pathogen uses multiple virulence factors to attack the host and alters them in the course of the infection. Furthermore, the presence of P. aeruginosa in the area of intense inflammation induces genotypic variation in the toxins, exoenzymes, secretion systems and surface structures, and thus, significant changes in the relationships between bacterial cells and host structures (Cigana et al., 2011; Lavoie et al., 2011). On the other hand, the impact of P. aeruginosa is characterised with numerous processes at molecular, cellular and organism levels and abundance of complex regulatory interactions. Despite that, Tak and Firestein (2001) reported that NF-кB is a crucial regulator of inflammation, and Koprowska and Czyz (2010) established the possibility for inhibition of NF-кB activity by parthenolide. On the basis of these facts, we have used the natural product Feverfew for control of inflammation in P. aeruginosa-infected dogs. The duration of intake was aimed at a moderate NF-кB inhibition. The resulting effect was proved by the lack of statistically significant deviations in NF-кB values in dogs receiving the phytopreparation (group II and III). This was also true for dogs from group I, where the variations in blood NF-кB values were insignificant between post infection days 7 and 10. Dogs from this group were on 10-day antibiotic therapy initiated on the 48th hour after the infection with P. aeruginosa, but the specific effect was observed on the 5th day of antibiotic therapy. Enrofloxacin penetrates well in tissues, has a continuous post antibiotic effect against P. aeruginosa (Sarkozy, 2001) and is effective against bacterial strains multiresistant to convenient antimicrobial drugs. The drug is also able to enhance the formation of neutrophil extracellular traps (Jerjomiceva et al., 2014).
NO appears to be an important element in the inflammatory process, being an endogenous regulator of vascular tone (Cirino et al., 2006; Tripathi et al., 2007). In healthy subjects its blood levels are influenced by the gender, age, and nutrition (Ghasemi and Zahediasl, 2011). In our experimental model, the impact of these factors was reduced to a minimum. In the present study, NO concentrations increased as early as the 4th hour after s.c. inoculation of P. aeruginosa (Table 1). This increase was most probably due to enhance NO production by cell elements at the inoculation site (Tripathi et al., 2007). Serum NO concentration after the 4th hour in dogs with antibiotic therapy (group I), as well as in dogs on phytotherapy with Feverfew were reduced. This could be due to the applied treatments, as well as to their effects on NF-kB (Figure 1), which is able to control NOS activity (Cirino et al., 2006). By the end of the study period – 14th day, serum NO concentrations were higher in all experimental dogs compared to controls (Table 1). This is a clear attestation for the role of NO in immune mechanisms associated with adaptive immunity. Tripathi et al. (2007) commented on the role of NO for specific immunity and discussed its influence on the Th1/Th2 ratio. The observed biphasic increase in serum NO concentrations in the early and late stage of infection development emphasise its significance for both non-specific and specific defense mechanisms. With this regard, the N/L ratio could also provide some information, although limited. Celikbilek et al. (2014) believe that N/L ratio is a simple and easily available marker of subclinical inflammation, while Ishimine et al. (2013) associated its alterations in combination with other parameters. Our data were comparable to those, showing a marked increase in N/L ratios between post infection hours 4 and 72 in all P. aeruginosa-infected dogs (Table 2). Dogs treated with Feverfew (group II) showed the most remarkable increase in N/L ratios, which could result from the effects of phytopreparation on blood circulation. Parthenolide (the major sesquiterpene lactone in medicinal plant Feverfew) action does not appear to be limited to a single mechanism. Plant extracts affect a wide variety of physiologic pathways. Some of these mechanisms have been discussed previously.
Conclusion and Clinical Implications
The inoculation of P. Aeruginosa in dogs provoked an inflammatory response accompanied with changes in serum NF-кB and NO concentrations, as well as of blood N/L ratio. These parameters could be utilised in clinical practice not only for characterisation of inflammatory response to skin infections. The use of Feverfew with active principle parthenolide, applied at the very beginning of infection/inflammation response inhibits effectively NF-кB, which justifies its application in dogs as anti-inflammatory agent. Extensive large-scale clinical investigations for elucidation of effects on host defense – innate and immune systems as other protective cellular responses are however needed. This approach does not guarantee eradication of the pathogen. The applied complementary therapy with antibiotic against the pathogen combined with phytotherapy for host response modulation in the tested experimental model, yields good and promising results.
ACKNOWLEDGEMENTS
Our heartiest thanks to Mrs Daniela Ivanova, Faculty of Veterinary Medicine, for her technical assistance in preparing this manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests in this manuscript.
REFERENCES





