Advances in Animal and Veterinary Sciences
Research Article
Relationship of in vitro Cervical Mucus Penetration Assay with IgA and IgG Type Antibodies in Cross-bred Cows
Vikrant Jarora, Vinod Kumar Gandotra, Ranjna Sandhey Cheema, Amrit Kaur Bansal, Shahbaz Singh Dhindsa
Department of Veterinary Gynaecology and Obstetrics, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana-141004, Punjab, India.
Abstract | IgG and IgA class antisperm antibodies were detected in cervical mucus of 25 crossbred cows with SperMar test. Sperm penetration through cervical mucus of these cows was also analysed in vitro. Relationship of in vitro CMPT with IgA and IgG type antibodies in cervical mucus was evaluated. Animals were grouped as G-1(>40% IgG, IgA) and G-II (< 40% IgG, IgA). IgG and IgA type antibodies were detected as 52.7±2.3%, 54.3±9.4% and 28.9±1.4%, 28.5±7.1 in cervical mucus of G-I and G-II, respectively. It indicated a significant (p<0.05) difference of 23.8% and 25.8% in IgG and IgA class antibodies in cervical mucus among the two groups. A total of 54.9%, 56.8% of the tested cows had significant level of IgG and IgA in cervical mucus respectively. Sperm penetration of cattle bull spermatozoa in cervical mucus of 25 cows showed a variation. A mean distance covered by spermatozoa/ 30 min and number of spermatozoa penetrated in peak 0.5 cm in cervical mucus was 15.3±1.8 mm and 356.8±84.9, which ranged from 0-40 mm and 0-1414 respectively. Higher percentage of IgG, IgA did not have negative effect on in vitro CMPT. Out of 25 cows, > 40% IgA and IgG were detected in 13 and 17 cows respectively. Among the cows with higher percentage of IgA/IgG, there were only 6 cows (28%) that had higher level of both IgA and IgG (>40%) and low values of CMPT. There were also one (3.8%) and four (15.3%) cows with only > 40% IgA and IgG and low values of CMPT, respectively. It can be concluded that higher percentage of IgG-ASA and IgA-ASA reduced in vitro penetration of spermatozoa through cervical mucus in 44% of the tested cross-bred cows. However, there was not any negative effect of ASA on in vitro CMPT in a group of cows with > 40% IgG/IgA in cervical mucus.
Keywords | Cross-bred cows, Cervical mucus, IgG, IgA, CMPT
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 15, 2014; Revised | November 12, 2014; Accepted | November 14, 2014; Published | November 26, 2014
*Correspondence | Ranjna Sandhey Cheema, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, India; Email: [email protected]
Citation | Jarora SV, Gandotra VK, Cheema RS, Bansal AK, Dhindsa SS(2014). Relationship of in vitro cervical mucus penetration assay with IgA and IgG type antibodies in cross-bred cows. Adv. Anim. Vet. Sci. 2 (11): 606-611.
DOI | http://dx.doi.org/10.14737/journal.aavs/2014/2.11.606.611
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2014 Jarora et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The cervical mucus (CM) functions as a guide for normal sperm to enter the uterus and may also act as a filter to prevent the passage of abnormal spermatozoa into the upper part of reproductive tract. Fertilizing capacity of spermatozoa has been shown to be strongly related to cervical mucus penetration test (CMPT) in bull (Tas et al., 2007) Impairment of cervical mucus penetration is an important component of the infertility effect of antisperm antibodies. Presence of IgA–antisperm antibodies (ASA), especially in cervical mucus is of great significance (Wang et al., 2009). ASA in seminal plasma or cervical mucus (CM) impairs the ability of sperm cells to penetrate cervical mucus in human (Eggert-krust et al., 1991) and anti-fertility effect of ASA may be mainly due to immobilization of sperm in the CM (Check et al., 1994). Investigations in veterinary field regarding the influence of ASA in CM on fertility rates are very few. Therefore, relationship of in vitro CMPT was analysed with IgA and IgG type antibodies, detected by SpermMar test in cervical mucus of cross-bred cows.
MATERIAL AND METHODS
Procurement of Cervical Mucus
Cervical mucus samples were collected from 25 cross-bred cows of dairy farm, Guru Angad Dev Veterinary and Animal Sciences University and private dairy farms around Ludhiana, Punjab, India. At the time of estrus, CM was collected with the help of artificial insemination (AI) pipette in sterilized vials, mildly sonicated at 20 watts, inactivated at 56o C for 30 min and stored in aliquots at -20o C till further use.
Procurement of Semen
Fifty frozen semen straws of each of two bulls were procured from Semen Bank Bhattian, Khanna, Punjab, India.
Indirect Sperm Mar Test with Sperm Mar kit
Indirect Sperm Mar test was performed as per the method of FertiPro (2011). Adherence of latex particles to different parts of spermatozoa in the presence of cervical mucus of cross-bred cow is shown in Fig 1. Diluted cervical mucus 1/4 with TALP medium, pH 7.4 and incubated at 37° C for 30 min, collected the motile sperms by centrifugation through histopaque, suspended the sperm pellet in TALP and adjusted the sperm concentration to 20 X 106. Incubated 100 µl of the sperm suspension of motile spermatozoa with 100 µl of inactivated 1/4 cervical mucus for 1hr at 37°C. Added 2 ml of TALP, mixed well and centrifuged for 10 min at 1500 rpm. Pellet was re-suspended with 50 µl of TALP. On a slide, mixed 10 µl of sperm suspension and 5 µl of Sperm Mar latex particles IgG / IgA, mixed, covered with cover slip, kept in humid chamber for 5 min and observed under bright field microscope at 400 X. Attachment of latex particles to the head / tail or whole sperm was observed. Adherence of latex particles to head, tail or both was noted and about 150 sperms in different fields were counted. Sum of all percent bound latex particle(s) from the various locations on the sperm surface per 100 spermatozoa was calculated:
%total bindino= (No. of sperms with bound lates particle/s)/(Totla no. of sperms counted) x 100
Cervical mucus penetration test (Murase and Braun, 1990)
CMPT was performed as per the method of Murase and Braun (1990) in CM of 25 cows using pooled frozen-thawed semen. Cervical mucus was filled in a capillary by capillary action. Capillary was sealed from one side with polyvinyl alcohol powder and pre-heated at 37o C for 10 min. Approximately 100 µl of frozen-thawed semen was placed at the bottom of an eppendorf tube and a capillary tube was placed with its open end in the semen. After 30 min of incubation at 37o C, the capillary tube was fixed on scaled glass slide and viewed under bright field microscope at 400 X. The length of the tube was then scanned to establish the distance furthest from the semen reservoir attained by spermatozoa. The maximum distance of migration of spermatozoa after 30 min of incubation was defined as the migration distance. Number of migrated spermatozoa was counted in the peak 0.5 cm.
Statistical analysis
In Sperm Mar test, 40% reaction between motile spermatozoa and coated latex particles of IgG and IgA is considered as lower limit of significant activity. Therefore animals were grouped as G-1(>40% IgG, IgA) and G-II (< 40% IgG, IgA). Relationship of IgA and IgG in cervical mucus with in vitro CMPT was analyzed statistically according to Independent Sample T-Test.
RESULTS AND DISCUSSION
Detection of IgG and IgA Type Antibodies by Sperm Mar Test
Binding of latex particles to head, tail or both was detected in the presence of blood serum or cervical mucus, which indicated the development of ASA against sperm surface proteins (Figure 1). IgG and IgA type antibodies were detected as 52.7±2.3 %, 54.3±9.4% and 28.9±1.4 %, 28.5±7.1 in G-I and in G-II, respectively (Table 1). It indicated a significant (p<0.05) difference of 23.8 % and 25.8% in IgG and IgA class antibodies in cervical mucus among the two groups. A total of 54.9 %, 56.8% of the tested cows had significant level of IgG and IgA in cervical mucus respectively. In general, the proportion of motile spermatozoa reacting in the SpermMar-IgA test is smaller than that reacting in the SpermMar-IgG test, but the contrary may occasionally occur (Ackerman et al., 1988), because IgA are of secretory type and less in quantity than IgG. In the present study, percentage of motile spermatozoa reacting in SpermMar-IgA and SpermMar-IgG test was almost same.
CMPT
Distance covered by spermatozoa in cervical mucus/30 min and number of spermatozoa penetrated in peak 0.5 cm was evaluated in CMPT. Sperm penetration of cattle bull spermatozoa in cervical mucus of 25 cows showed a variation. A mean distance covered by spermatozoa/ 30 min and number of spermatozoa penetrated in peak 0.5 cm in cervical mucus was 15.3±1.8 mm and 356.8±84.9, which ranged from 0-40 mm and 0-1414 respectively.
Relationship of IgA/IgG and CMPT
Since presence of ASA can inhibit passage of spermatozoa through cervical mucus, prevent membrane fluidity changes needed for capacitation, reduce the ability of spermatozoa to undergo the acrosome reaction, and interfere with binding to the zona pellucida and fertilization (Fijak and Meinhardt, 2006). Therefore, IgA and IgG in G-I and G-II were statistically compared with distance covered by spermatozoa in cervical mucus/30 min and number of spermatozoa penetrated in peak 0.5 cm in CMPT (Table 1). Distance covered by spermatozoa / 30 min was non-significantly (p<0.05) higher in cows with > 40% IgG, IgA (17.2±2.6, 19.2±2.6) than cows with < 40% IgG, IgA (12.5±2.1, 11.1±1.9). Number of spermatozoa penetrated in peak 0.5 cm was also higher in cows with >40% IGg, IgA (471.6±135.8, 408.7±124.4) than cows with < 40% IgG, IgA (232.3±87.9, 278.9±104.5), respectively. It indicated that overall higher percentage of IgG, IgA did not have negative effect on in vitro CMPT. Studies done in human also revealed variable effect of ASA on the acrosome reaction/ capacitation (Fijak and Meinhardt, 2006) and sperm binding to human zona pellucidae (Bronson et al., 1982; Bronson et al., 1990a; Bronson et al., 1990b). Presence of ASA can inhibit passage of spermatozoa through cervical mucus, prevent membrane fluidity changes needed for capacitation, reduce the ability of spermatozoa to undergo the acrosome reaction, and interfere with binding to the zona pellucida and fertilization (Fijak and Meinhardt, 2006). It can be predicted that although IgG and IgA were higher in animals but did not interfere with the cervical mucus penetration but may have interference with other processes i.e. capacitation, binding to zona pellucida or fertilization.
Correlation between in vitro CMPT and Cervical Mucus-IgA and IgG
Although, higher percentage of IgG/IgA in G-I did not show any negative effect on CMPT, but a positive Pearson correlation of 0.26/0.16; 0.48/0.28 was obtained between distance covered by spermatozoa in 30 min/ spermatozoa penetrated in peak 0.5 cm and IgG; IgA respectively. Out of 25 cows, > 40% IgA and IgG were detected in 13 and 17 cows respectively. Among the cows with higher percentage of IgA/IgG, there were only 6 cows (28%) that had higher level of both IgA and IgG (>40%) and low values of parameters of CMPT (Table 2). There were also one (3.8%) and four (15.3%) cows with only > 40 % IgA and IgG and low values of CMPT, respectively. It can be interpreted from this observation that ASA developed in cervical mucus of cross bred cows were of immobilizing type in these cows. Antibodies in the cervical mucus bind to the antigens on the sperm surface and due to antigen-antibody reaction, head to head, head to tail or tail to tail agglutination occurs. Therefore, it indicated that IgA and IgG in cervical mucus may reduce the penetration of spermatozoa through cervical mucus by immobilizing or agglutinating the spermatozoa. Study carried out by Wang et al. (2009) corroborated that presence of IgA-ASA, especially in cervical mucus is of great significance because the attachment of IgA antibodies to the sperm surface lowers sperm penetration ability, while IgG antibodies did not have the same effect. On the contrary our study revealed that IgA and IgG in cervical mucus are of equal importance in decreasing the penetration ability of spermatozoa. There is strong evidence that in humans, sperm-mucus interactions can be affected by local ASA, especially of the IgA class, both under in vitro and in vivo conditions (Eggert-Kruse et al., 1991). ASA in seminal plasma or CM impaired the ability of sperm cells to penetrate cervical mucus (Eggert-Kruse et al., 1995). Check et al. (1994) demonstrated that the anti-fertility effect of ASA may be mainly due to immobilization of sperm in the CM. ASA have been shown to react with plasma membrane with variable biological effects (Chamley and Clarke, 2007).
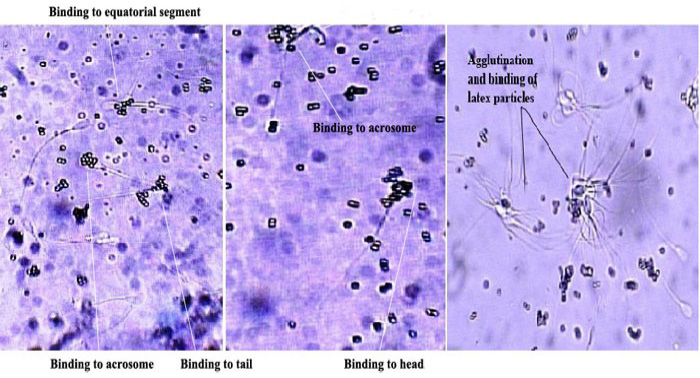
Figure 1: Showing binding of IgG/ IgA latex particles to spermatozoa in the presence of cervical mucus of a repeat breeding cow
Table 1: Relationship of IgA and IgG class antibodies in cervical mucus with cervical mucus penetration test
|
Type of Antibody |
Group Number |
Percentage (range) |
CMPT |
|
|
Distance (mm)/30 min |
Sperm count in peak 0.5 cm |
|||
|
IgA (%) |
G-I ( n =15) |
45.69±2.95a (30.7-57.6) |
19.2±2.6a (2.0-28) |
471.6±135.8a (0-1414) |
|
G-II (n=10) |
21.05±2.32b (17-37.1) |
11.1±1.9a (0-23) |
232.3±87.9a (0-1414) |
|
|
IgG (%) |
G-I (n =15) |
56.9±3.8a (42.8-88.1) |
17.2±2.6a (0-28) |
408.7±124.4a (0-1414) |
|
G-II ( n=10) |
31.75±1.63b (24.2-36.5) |
12.5±2.1a (0-23) |
278.9±104.5a (0-1114) |
|
Values with different superscripts are significant (p<0.05)
Table 2: Status of IgA and IgG type antibodies and CMPT in cross bred cows
|
S. No. |
Cow No. |
IgA (%) |
IgG (%) |
CMPT |
|
|
Distance covered by spermatozoa/30 min |
Number of spermatozoa penetrated in peak 0.5 cm |
||||
|
1. |
1359** |
43.1 |
439 |
11 |
77 |
|
2. |
1214 |
57.6 |
88.1 |
16 |
380 |
|
3. |
1383* |
36 |
44.4 |
16 |
15 |
|
4. |
1294** |
57.6 |
88.1 |
11 |
110 |
|
5. |
1358 |
20. 6 |
32.9 |
23 |
230 |
|
6. |
1348 |
25.3 |
36.5 |
11 |
75 |
|
7. |
1392* |
18.6 |
54.4 |
10 |
274 |
|
8. |
1391 |
30.4 |
32.3 |
10 |
89 |
|
9. |
1396** |
45.4 |
49.5 |
2 |
0 |
|
10. |
1402** |
47.8 |
50 |
17 |
230 |
|
11. |
1355*** |
49.4 |
27.1 |
18 |
290 |
|
12. |
1271 |
17 |
35 |
11 |
321 |
|
13. |
1129 |
40.2 |
51 |
22 |
1291 |
|
14. |
1386 |
69 |
63.2 |
40 |
1414 |
|
15. |
1399 |
25 |
35.8 |
10 |
89 |
|
16. |
1380** |
54 |
53.9 |
15 |
115 |
|
17. |
KK7** |
49.2 |
40.7 |
20 |
49 |
|
18. |
1959* |
29.1 |
42.2 |
0 |
0 |
|
19. |
V- 155 |
40.5 |
61 |
28 |
1091 |
|
20. |
151 |
32.3 |
25.6 |
10 |
501 |
|
21. |
2199* |
34 |
40 |
0 |
0 |
|
22. |
135 |
49.5 |
43.6 |
20 |
508 |
|
23. |
5206 |
37.1 |
28.7 |
10 |
80 |
|
24. |
8-JAS |
56.8 |
66.1 |
30 |
577 |
|
25. |
44-JAS |
32.5 |
24.2 |
22 |
1114 |
>40% IgG and <25 mm distance covered by spermatozoa in 30 min/<350 spermatozoa penetrated in peak 0.5 cm; **> 40% IgA/ IgG and <25 mm distance covered by spermatozoa in 30 min/<350 spermatozoa penetrated in peak 0.5 cm and *** >40% IgA and <25 mm distance covered by spermatozoa in 30 min/<350 spermatozoa penetrated in peak 0.5 cm
Therefore, it can be concluded that higher percentage of IgG-ASA and IgA-ASA reduced in vitro penetration of spermatozoa through cervical mucus in 44% of the tested cross-bred cows, however, there was not any negative effect of ASA on in vitro CMPT in a group of cows with > 40% IgG/IgA in cervical mucus.
ACKNOWLEDGEMENTS
Authors are thankful to UGC, New Delhi for providing grant under major research projects. Authors are also thankful to semen freezing lab, GADVASU, Ludhiana, India for providing semen samples.
REFERENCES





