Advances in Animal and Veterinary Sciences
Research Article
Taenia Hydatigena from Natural Infection in Small Ruminants to Experimentally Infected Dogs in Egypt
Mohamed El-Beskawy*, Wael Felefel, Mohamed Morsi Elkamshishi
Faculty of Veterinary Medicine, Matrouh University, Egypt.
Abstract | Cysticercus tenuicollis (C. tenuicollis) bubble-like metacestode is the larval stage of Taenia hydatigena (T. hydatigena). This larval stage was found in different numbers with various sizes from one cm up to 6–8 cm, which attached to the omentum, mesentery, and liver surface among domestic intermediate ruminant hosts. Prevalance of C. tenuicollis at Matrouh abattoir was 387(19.5%) cases out of 1986, 318 out of 1431 (22%) in goat compared to 69 out of 555 (12%) of sheep. Studying the effect of different viability duration days of C. tenuicollis cysts was estimated and different infective dosage of C. tenuicollis cysts to yield defined numbers of T. hydatigena adult worms in the small intestine of experimentally infected dogs. The study was carried out through faecal samples diagnosis, administration of C. tenuicollis cysts and measurement of T. hydatigena adult worms. Administration of 54 C. tenuicollis cysts to 36 healthy labeled experimental dogs. The healthy experimental dogs were divided according to C. tenuicollis cysts viability and different dosage into 3 groups; Group 1 (viability within 24 hours), Group 2 (viability within 48 hours), and Group 3 (viability within 96 hours) and each viability groups were divided into two dosage one or two C. tenuicollis cysts which contains each dosage six experimental dogs three females and three males. The results indicated that the over all of total infected dogs were 16/36 (44.44%) experimentally infected with C. tenuicollis and the females were higher than males as 10/16 (62.5%) were females. the infection was 100% within 24 hours (group1) with two cysts infective dose among female only with the shorter average length than males and overall infection among within 24 (group 1) was 8/12(66.66%). On other hand, the low infection rate to yield adult worms among viability duration within 96 (group 3), was 3/12 (25%) but the infection rate within 48 (group2) was 5(41.66%) out of 12 experimental infected dogs So, it could be concluded that, the risk of infection of Taenia hydatigena adult worm among experimental dogs was belonged to infective dosage two C. tenuicollis cysts with viability duration within 24 hours.so, prevention of dogs from consumption of abattoir offal’s within 24 hours, or before treatment is a must in controlling the disease.
Keywords | Cysticercus tenuicollis, Taenia hydatigena, Experimental dogs, Egypt
Received | April 17, 2021; Accepted | April 25, 2021; Published | September 15, 2021
*Correspondence | Mohamed El-Beskawy, Faculty of Veterinary Medicine, Matrouh University, Egypt; Email: [email protected]
Citation | El-Beskawy M, Felefel W, Elkamshishi MM (2021). Taenia hydatigena from natural infection in small ruminants to experimentally infected dogs in egypt. Adv. Anim. Vet. Sci. 9(10): 1732-1738.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1732.1738
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 El-Beskawy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
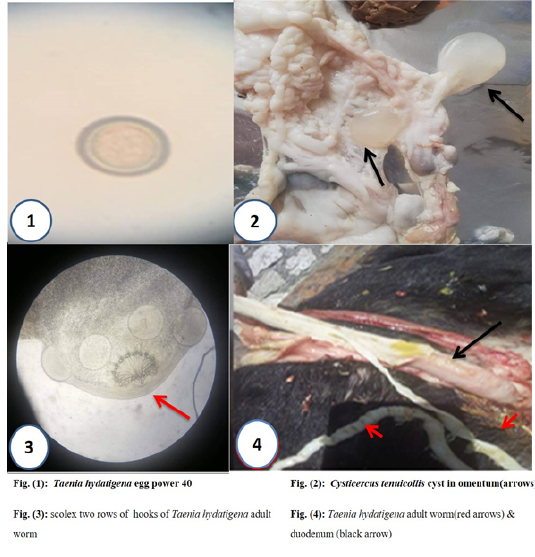
Cysticercus tenuicollis (C. tenuicollis) bubble-like metacestode is the larval stage of Taenia hydatigena was found in different numbers with varies sizes from one cm up to 6–8 cm, which attached to the omentum, mesentery, and liver surface among domestic intermediate ruminant hosts as sheep, goats, and cattle. It the infective stage to final host the canines such as dogs and fox through digestion the C. tenuicollis cyst to yield adult worm after incubation period 51 days in the small intestine (duodenum) of the final host. (Behrestaghi et al., 2018), however, they can also be found in the lungs, heart, uterus or kidneys (Gomez-Puerta et al., 2015).
Taenia hydatigena has a global distribution with a prevalence range of 0.1–32.0%, differing between countries and hosts (Braae et al., 2015; Nguyen et al., 2016), prevalence is usually higher in sheep and goats in most African and European countries compared to countries in Asia (Braae et al., 2015; Scala et al., 2015).
In Egypt, the infection rates of C. tenuicollis were 16% and 19% in sheep and goat samples, respectively, and the higher prevalence in females than in males, especially those above two years of age infected was higher infection rate than younger animals (Mosaab Adl Eldin Omar et al., 2016).
The financial impact of T. hydatigena is less important but during their time in the liver, larval stages of T. hydatigena may cause tissue damage leading to Condemnation of infected organs. (Jenkins et al.,2014), in addition, migration of the larvae can result in traumatic hepatitis, resulting in the death of the host animal (Rostami et al., 2013).
Infection with C. tenuicollis favors infection and growth of pathogenic microorganisms that can cause necrotic hepatitis, which gives rise to economic losses associated with reduced productivity among the affected animals and condemnation of damaged organs (Popova et al., 2013). In high infections, causing mortality in 5 of 21 (23.8%) female lambs, in central Sardinia, Italy (Scala et al., 2016)
Cysticercus tenuicollis cysts are round and white in color but in some cases, they are yellowish and surrounded by a semi-transparent wall composed of fibrous tissue formed from body host, which filled with fluid contains a high amount of protein (93.4 nm), carbohydrates, and other essential elements, which absorbed by the parasite lead to the cyst was infective for long duration in external environmental (Essa et al., 2011).
Thus, this work aimed to estimate the effect of different viability duration days of C.tenuicollis cysts obtained from slaughtered sheep and goat from General Mersa Matrouh abattoir at Mersa Matrouh Governorate-Egypt and different infective dosage of C.tenuicollis cysts to yield defined numbers of T. hydatigena adult worms in the small intestine of experimentally infected dogs.
MATeRIAL AND METHODS
Study design
An experimental blinded randomized comparative trial was done to compare the estimated effect of a different viability duration days with a different infective dosage of C.tenuicollis cysts to yield defined numbers of T. hydatigena adult worms in the small intestine of experimental dogs. The experimental dogs were trapped in registered place under control of Ministry of Supply and Internal Trade, Alexandria Governorate, Egypt; registered number was 584813328 through three months from January 20 to April 20, 2020. The inclusion criteria were experimental dogs from both sexes with different age categories, free from infection with T. hydatigena adult worms. All respective animal protocols were reviewed by state ethics commission and have been approved by competent authority (Ethical committee FWA No: 00018699 and IRB No: 00012098, faculty of medicine, Alexandria university, Egypt).
Samples
Faecal samples: Faecal samples from all 40 dogs to exclude the infected dog with T. hydatigena adult worms by detection of the characteristic egg, by Merthiolate iodine formaldehyde concentration technique (MIFC) then from 36 dogs experimentally infected by cyst of T. hydatigena at different intervals of 41, 47 and 57 days post infection by cysts. (Jayewardeve, 1957).
Cyst samples: One hundred C.tenuicollis cysts were collected from sheep and goats at General Mersa Matrouh abattoir, Matrouh, Egypt, from omentum and liver then washed with normal saline and preserved in cubs contains normal saline in refrigerator at average temperature 6°C±2°C centigrade with 60-80% humidity. From which 54 C. tenuicollis cysts have long-necked single scolex, virtually translucent cyst fluid was administrated to 36 healthy labeled dogs Table (1). At animal house of Faculty of Agriculture-Alexandria University in Alexandria governorate
Animals
Forty stray dogs were collected randomly from center and west Alexandria governorate, Egypt and were kept at animal house of Faculty of Agriculture-Alexandria University, Egypt. Those animals were clinically examined according to Kelly 1991 including (body weight, age, temperature, pulse, and Capillary Refill Time CRT).
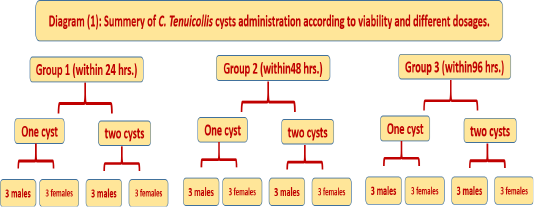
Administration of 54 C. tenuicollis cysts to 36 healthy labeled dogs which were divided according to C. tenuicollis cysts viability and different dosage into 3 groups as following:
Group 1 (viability within 24 hours): This group included the 12 healthy labeled dogs with equal divided sex. Then divided the 12 dogs according to administer a 2 difference number of C. tenuicollis cysts dosage as follows (one, two) each dosage feed by 6 dogs; 3 male and 3 female, within 24 hours post collection from abattoir. Table (1) and diagram (1).
Group 2 (viability within 48 hours): This group included the 12 healthy labeled dogs with equal divided sex. Then divided the 12 dogs according to administer a 2 difference
Table 1: Shows the summery of Cysticercus tenuicollis cysts administration according to viability and different dosages.
| Groups | No. of Cysticercus tenuicollis Cysts | Animal sex | Total | |
| Male | Female | |||
| Group (1) within 24 hrs. | one cyst | 3 | 3 | 6 |
| two cysts | 3 | 3 | 6 | |
| Group (2) within 48 hrs. | one cyst | 3 | 3 | 6 |
| two cysts | 3 | 3 | 6 | |
| Group (3) within 96 hrs. | one cyst | 3 | 3 | 6 |
| two cysts | 3 | 3 | 6 | |
| Total | 54 cysts | 18 | 18 |
36 animals |
number of C. tenuicollis cysts dosage as follows (one, two) each dosage feed by 6 dogs; 3 male and 3 female, within 48 hours post collection from abattoir. Table (1) and diagram (1).
Group 3 (viability within 96 hours): This group included the 12 healthy labeled dogs with equal divided sex. Then divided the 12 dogs according to administer a 2 difference number of C. tenuicollis cysts dosage as follows (one, two) each dosage feed by 6 dogs; 3 male and 3 female, within 96 hours post collection from abattoir. Table (1) and diagram (1).
All C. tenuicollis cysts were administrated to healthy dogs must have long-necked single scolex, virtually translucent cyst fluid. (Singh et al., 2015).
Microscopical examination
All obtained cysts were examined for identifying the characteristic of scolexes and examination of head of adult worm for complete identification, also fecal samples from all 40 dogs to exclude the infestation by T. hydatigena and other internal parasites, in addition to 36 experimentally infected dogs, fecal examination by Merthiolate iodine formaldehyde concentration technique (MIFC). (Fatma EL-Lessy et al., 2019) At 3 times after Administration C. tenuicollis cysts to detect the characteristic egg of T. hydatigena and/or segments of adult worms in the fecal matter of the examined dogs at 41, 47 and 57 days after administration C. tenuicollis cysts respectively.
Surgical interference and anesthesia
This step was done to 16 experimental dogs that had positive fecal matter examination for T. hydatigena adult worms eggs by abdominal section laparotomy operation. (Bednarski et al., 2011) for the detection of the adult worm in the duodenum of the small intestine of dogs through cephalic vein injected xylazine 2% (XYLA-JECT ® Adwia Pharmaceuticals at dose 0.15 ml / 1 kg body weight. combination of ketamine 5% at dose from 50 to100 mg (from 1ml to 2 ml) per each 16 labeled dogs (Bednarski et al., 2011).(Siddiqui et al., 2012; Lysimachos et al., 2015).
The duodenum of the small intestine of experimentally infected dogs were opened and worms were collected and saved in 70% ethanol for morphological features examination for measuring the length and the scolex, identifying hooks shape and number of rows (Faraj A et al., 2018).
Post-operative experimental dogs’ management
All 36 experimental dogs after abdominal section laparotomy operation were lifted for recovered from anesthesia then all experimental dogs were administrated Praziquantel a drug name was “BILTRICIDE “600 MG 4 TAB (Produced by: Alexandria Co. for Pharmaceuticals – Alexandria – A.R.E) under 10mg/kg in single dose then all experimental dogs its fecal matter were examined by Merthiolate iodine formaldehyde concentration technique (MIFC) after treatment to confirm that all experimental dogs were enrolled in present study free from helminthes intestinal infection.
Macroscopical examination to adult worm
Adult worm examination for measuring the length and counting numbers of adult worms per each animal. (Faraj A et al., 2018) after administration of C. tenuicollis cysts to all 36 experimentally infected dogs. During 51 days the incubation period. (Gomez-Puerta L et al., 2019) dogs were fed a basic standard food and water to ensure they did not get any infection with other worms.
Statistical Analysis
The data succumbed to the Statistical Analysis System
Table 2: Distribution of Cysticercus tenuicollis (Taenia hydatigena cysts) in sheep and goats
| Species | No .of examined animals | No .of Infected animals | No. of Taenia hydatigena cysts per animal at abattoir | ||||||
| 1 cyst | 2 cysts | 3 cysts | 4 cysts | 5 cysts | 5-10 cysts | >10 cysts | |||
| Sheep | 555 | 69 | 32 | 21 | 5 | 1 | 2 | 4 | 4 |
| Goats | 1431 | 318 | 229 | 57 | 15 | 2 | 1 | 3 | 11 |
| Total | 1986 | 387 | 261 | 78 | 20 | 3 | 3 | 7 |
15 |
Table 3: Shows the number and length of Taenia hydatigena adult worm yielded according to the number of given Cysticercus tenuicollis
| Groups | No. of Cysticercus tenuicollis Cysts | Animal sex | Number of obtained Taenia hydatigena adult worms | ||||
| Male | Female | Male | Female | ||||
| No. | Average length | No. | Average length | ||||
| Group (1) within 24 hrs. | one cyst | 3 | 3 | 1 | 120 cm | 2 | 55cm |
| two cysts | 3 | 3 | 2 | 39cm | 3 | 14.4cm | |
| Group (2) within 48 hrs. | one cyst | 3 | 3 | 1 | 85cm | 1 | 107cm |
| two cysts | 3 | 3 | 1 | 90 cm | 2 | 42cm | |
| Group (3) within 96 hrs. | one cyst | 3 | 3 | 0 | 0 | 1 | 94 cm |
| two cysts | 3 | 3 | 1 | 102cm | 1 | 101cm | |
| Total | 54 cysts | 18 | 18 | 6 | 10 | ||
SPSS version 22 by used ROC curve, one way ANOVA test, Pearson Correlation and Kaplan Meier Survival curve. (Allen P, IBM United States Software Announcement, 2014).
RESULTS
The total positive infected experimental dogs were 16 (44.44%) out of 36 dogs experimentally infected with C. tenuicollis and the females were higher than males as 10 (62.5%) out of 16 infected dogs were females, but the difference statistically insignificant (chi-square x2= 1.800, P = 0.180) with odd ratio value was 0.4 with CL 95% (0.104-1.544). The infection rate was 50% and 83.33% within 24 hours (group 1) with one cyst and two infective dosages respectively among both sex. with the longest average length 120 cm in one cyst infected male.
the infection rate was 50% and 83.3% within 24 hours (group 1) with one cyst and two infective dose respectively among both sex. with the longest average length 120 cm in one cyst infected male.
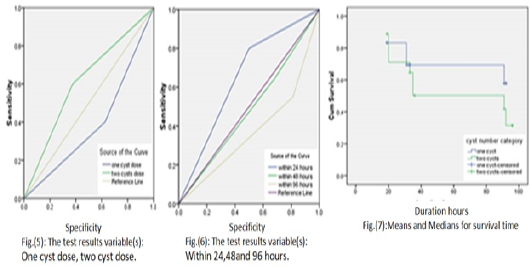
Insignificant differences among difference given C. tenuicollis cysts number within difference viability duration hours which by one way ANOVA test which the p value was 1.000 and also insignificant differences between the C. tenuicollis cysts viability duration hours among difference infective cysts dosage by Kaplan Meier Survival curve
was reported that; Log Rank (Mantel-Cox) was p value = 0.467,Breslow (Generalized Wilcoxon) was p value = 0 .748 and Tarone-Ware p value = 0.610 which revealed that the minimum viability duration of C. tenuicollis cyst was 60.259 hours which belong to two C. tenuicollis cysts infective dose then 71.245 hours belong to one C. tenuicollis cyst infective dose and finely overall average mean viability duration of C. tenuicollis was 65.355 hours so the Kaplan Meier Survival curve was insufficient test to predict infection occur through difference cysts dosage within difference viability duration or so the receiver operating characteristic ROC curve was done for each research aspect Table (3) and Figure 5, 6 and 7.
ROC curve value was 0.612 belong to the two C. tenuicollis cysts versus one C. tenuicollis cyst the ROC curve value was 0.388 on other hand as regard to viability duration the highest ROC curve value was 0.650 belong to viability duration of C. tenuicollis cysts within 24 hours on other hand the rest of viability durations within 48 hours and 96 hours the ROC curve value were 0.481 and 0.369 respectively. Figure 5.
The correlation between the number of C. tenuicollis cyst and T.hydatigena adult worm yield which the Pearson Correlation value = 0 .368 with p value = 0.027
DISCUSSION
In the present study, the distribution of C. tenuicollis in goat was 318 out of 1431 (22%) compared to 69 out of 555 (12%) of sheep, reflecting that the nature of grazing of goats around the population of dogs at villages borders, versus to sheep grazing in the fields or deserts with less contact of dogs, so goat are at risk more than sheep. C. tenuicollis numbers per animals ranged from one cyst (67%), two cysts (20%) to more than ten cysts (0.03%), giving announcement about the possible infective dose to complete the life cycle of T. hydatigena.and from that data we designed the experiment on the predictive dose of one cyst and two cysts only with different three viability periods (24, 48 and 96 hours).while In Greek slaughtered sheep, the infection level due to C. tenuicollis was 29.4%. The infection levels in small ruminant carcasses in Brazil was 32% (Morais, et al., 2017).
The total infected experimental dogs with T. hydatigena adult worm were 16 (44.44%) out of 36 dogs experimentally infected with C. tenuicollis and the females were higher than males as 10 (62.5%) infected out of 16 infected dogs were females. but the gender wasn’t consider a risk factors for experimental dogs due to the odd ratio was OR = 0.4 CL 95% (0.104-1.544) but females experimental dogs were higher which contributed to which contributed to the male dogs less activity than normal due to all experimental dogs were cared inside animal house confirmed by Bentounsi et al. (2009) who reported that the activity of dogs are modify the structure of cestode community it has been speculated that T. multiceps is more expected in sheep dogs, and that T. pisiformis in hunting dogs T. hydatigena and E. granulosus in butcher’s dogs and also said that; male dogs are more likely to be infecting and dogs allowed to roam are more likely to be infecting (Bentounsi et al., 2009).
In current study, it was found that; the infection rate was 50% and 83.33% within 24 hours (group 1) with one cyst and two infective doses respectively among both sexes. With the longest average length 120 cm in one cyst infected among male due to present of one adult worm only in small intestine of experimental male dog and the viability of C. tenuicollis confirmed by confirmed by Al-Bayati (2012) that found the parasite has various specific cell which may related to the nature of this parasite and at last the total nature of parasitic bladders have a wide range of similarities with known connective tissue and its constituents of various cells, fibrous and other matrix components (Al-Bayati et al., 2012) Also, the results among all viability categories with 48 hours infective dosage with average length in female was 107 cm in one cyst infective dosage versus 90 cm in male in two cysts infective dosage and the adult worm was 102 cm among female in two cysts infective dosage belonged to (group 3) within 96 hours. this agrees with previous studies by Ibrahim et al. (2018) who revealed that the T. hydatigena adult worm recover at necropsy from the dogs and the infection is 100% in the first group where C. tenuicollis cyst was treated with ginger extract 62.5mg/ml and control group where C. tenuicollis cyst was non-treated. Regard worm length the same author found that the length obtained from worms of group no.4 ranged between 7–34 cm while the length of worms collected from the control group ranged between 9-86 cm (Ibrahim et al., 2018).
The length of adult worms vary viability was contributed to the different incubation period and the number present in duodenum of small intestine in dogs which In present study was reported that the incubation period for first time T. hydatigena eggs diagnosed in fecal matter was 57 days after experimental dog’s infection. So by Alvarez Rojas (2018) reported that; A the prepatent period for T. hydatigena was 42 to 56 days, on other hand Ibrahim et al. (2018) found that the incubation period is 40 days. (Ibrahim et al., 2018; Alvarez Rojas, 2018).
the present work reported that the minimum viability duration of C. tenuicollis was 54.125 hours which belong to 10 C. tenuicollis cyst infective dose then 61.924 hours belong to 5 C. tenuicollis cyst infective dose and finely 69.0 hours belong to 2 C. tenuicollis cyst infective dose which that belong to high amount of protein (93.4 nm), Carbohydrates and other essential elements which absorbed by the parasite in the liquid of the cyst so takes nourishments from this liquid (Essa et al., 2011).
The correlation between the number of C. tenuicollis cyst and T. hydatigena adult worm yield was positive strong so when increase the number of C. tenuicollis cysts were ate by final host corresponding increase yield T. hydatigena adult worm inside duodenum of small intestine among dogs which was confirmed by Ibrahim et al. (2018) who stated that when fed the dogs fresh viability C. tenuicollis among group (1) 8 cysts each dog it was yield 8 T. hydatigena adult worm which contributed by Gessese et al. (2015) supposed that such variation in the infectious rates may be due to geographical, environmental and meteorological factors such as climate, soil properties, relative humidity and season and rainfall level which are crucial to the life cycle of the helminths, the degree of pasture contamination and the way of raising and grazing of these animals which may favor the transmission cycle between ruminants and dogs. Other factors play subsidiary but important roles like animal management, feeding habits and grazing pattern followed in the local pasture which ultimately decrease the infection rates. However, although massive infections with e.g. T. hydatigena can cause severe disease and mortality in sheep, there are few indications that latent cysticercosis has obvious effects on the productivity of sheep and goats. (Ibrahim et al. 2018; Gessese et al., 2015).
Finally, in the existing study was discovered that; the highest ROC curve value belong to 10 C. tenuicollis cysts dose among all viability duration categories were 0.682 which was indicated the sufficient dose to get infection but the other C. tenuicollis cysts dosage were ROC the curve values less than this value which was indicated not sufficient dose to get the infection but the two C. tenuicollis cysts dose among group 1 viability within 24 hours it was considered the risky dose especially in Mersa Matrouh governorate due to infected small ruminant animals with C. tenuicollis cysts in general Mersa Matrouh abattoir from 27/11/2019 to 27/5/2020 were 387(19.5%) cases out of 1986 and within the infected cases were found that the slaughtered carcasses were infected with 2 cysts 78 (20.15%) cases versus 15 (3.87%) cases were infected with ten cysts and also when viewed that group 1 viability within 24 hours was the most risky viability duration due to the highest values of ROC 0.605 curve than other viability groups which was indicated the viability duration within 24 hours was sufficient factors and risky to get infection.
CONCLUSION
In conclusion, the risk of infection of Taenia hydatigena adult worm among dogs was belonged to infective dosage of C. tenuicollis cysts the viability duration within 24 hours with highly risk dose was two C. tenuicollis cysts. So, prevention of dogs from consumption of abattoir offal’s within 24 hours, or before treatment is a must in controlling the disease.
acknowledgements
The authors would like to thank Dr Sabreen Fadl assistant professor of biochemistry for her help during practical part of the manuscript.
conflict of interest
All authors declare that there is no any conflict of interest.
authors contribution
All authors approved the manuscript for publication.
REFERENCES