Advances in Animal and Veterinary Sciences
Research Article
Morphological Comparison in Spermatozoa from Italian and Egyptian Buffalo Bulls, and their IVF Outcome
Al Shimaa Al Housiny Hasab El-Naby1, Mohamed Mahmoud Moustafa Kandiel1,Karima Ghonaimy Mohamed Mahmoud2*, Gamal A. El-Sisy2, Walid Tawfik Mohamed Soliman3, Yousef Fawzy Ahmed2
1Department of Theriogenology, Faculty of Veterinary Medicine, Benha University, Egypt; 2Department of Animal Reproduction and A. I., National Research Center, Dokki, Giza, Egypt; 3Veterinary Department, Egyptian Armed Forces, Nasr city, Cairo, Egypt.
Abstract | Our study aimed at addressing the question “Do spermatozoa biometric measures prove its fertility potential?”. Therefore, we carried out a comparative study of the buffalo bull’s fertility potential between Italian and Egyptian breeds based on morphometric analysis of frozen-thawed semen and in vitro fertilization. Semen was evaluated post-thawing for plasma membrane integrity, morphology, and motility. Sperm morphology was quantified using computer-assisted evaluation. The cleavage rates, morula, and blastocyst morphologies were recorded for the in vitro assessment of the fertilizing capacity. Our results show substantial differences in motility, viability, abnormalities, plasma membrane (P<0.001), and acrosome integrities (P<0.01) between the two bull breeds. Spermatozoa of Egyptian bulls are characterized by a longer head length, larger head area and a higher ellipticity. In contrast, Italian buffalo spermatozoa are characterized by a wider head base and acrosomal width, larger head perimeter, higher rugosity, and difference in head shape. The total flagellum and principal piece lengths are longer in Italian bull sperm. Midpiece (volume and width) and principal piece (volume) measures are increased in Egyptian bull sperm. In contrast, the blastocyst rate was significantly (P< 0.05) higher in Italian than Egyptian bull semen. There is no marked difference between the two breeds in cleavage and morula rates. In conclusion, the detected considerable variations in sperm biometry between the Egyptian and Italian semen/spermatozoa are attributed to the significant variations in the in vitro fertility potentials.
Keywords | Biometry, Egyptian bulls, Italian semen, Spermatozoa, IVF.
Received | April 23, 2021; Accepted | May 06, 2021; Published | July 28, 2021
*Correspondence | Karima Ghonaimy Mohamed Mahmoud, Department of Animal Reproduction and A. I., National Research Center, Dokki, Giza, Egypt; Email: [email protected]
Citation | El-Naby SHH, Kandiel MMM, Mahmoud KGM, El-Sisy GA, Soliman WTM, Ahmed YF (2021). Morphological comparison in spermatozoa from italian and egyptian buffalo bulls, and their ivf outcome. Adv. Anim. Vet. Sci. 9(9): 1449-1455.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1449.1455
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mahmoud et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Italian frozen semen gained high popularity in the Egyptian market for the enhancement of farm animal productivity (especially milk yield), and genetic improvement. The Italian buffaloes show a kind of “purity”, result of obvious morphological and functional differentiation. The fertility of Italian frozen-thawed semen is better than that of the Egyptian breed and the reason for that variation is still elusive.
Various laboratory diagnostic tests are adopted for the evaluation of the bull breeding potency and soundness, such as a conventional semen picture, zonapellucida binding test, in vitro fertilizing ability, and acrosomal integrity assay (Abdel Dayem et al., 2009). Variations among bulls in artificial insemination (AI) centers concerning the non-return rates variations as much as 25%, and these differences cannot be detected using the routine semen analysis (Larson and Miller, 2000). Conventional semen analysis protocol essentially reveals clinical data regarding spermatogenesis, accessory sex glands activity and the functional capability of spermatozoa (Moazzam et al., 2015). Due to the possible role of sperm biometry in assessment of sire fertility (Casey et al., 1997), the application of head morphology and sperm morphometry into a fertility index could help in sire ranking challenged with their fertilizing capacity. The differences in metabolic characteristics of the sperm cell (Brackett and Oliphant, 1975), the ratio of sperm number to seminal plasma volume (Fukui et al., 1988), seminal plasma content and, plasma membrane composition of sperm (Alvarez and Storey, 1995) are employed to explain the variation in pregnancy or in vitro fertilization (IVF) rates between sires.
Previous reports in bovine (Roy, 2014) and equine (Phetudomsinsuk et al., 2008) demonstrated noticeable dissimilarities in sperm biometry among the breeds. Moreover, head dimensions(length, width, area, and perimeter) were markedly larger in sub-fertile than fertile stallions (Casey et al., 1997).
The quality of the frozen semen is the most influential factor that affects its fertility and conception rate. Freezability and fertility of frozen buffalo semen are lower than those of cattle (Kumaresan et al., 2005). In 2003, the Egyptian Ministry of Agriculture imported Italian buffalo frozen semen to improve the quality of Egyptian buffaloes (Fooda et al., 2011).
The present study aimed to distinguish the differences in semen features, sperm morphometry, and in vitro fertilization capability between Egyptian and Italian buffaloes’ breeds.
Material and Methods
All the procedures were accomplished according to the Ethics for Humane Treatment of Animal Use in Research Guidelines and abided by the relevant legislation of the Faculty of Veterinary Medicine at Benha University, Egypt (Ref. No. BUFVTM06-09-2019).
Semen source
Two types of frozen semen were used, including Italian and Egyptian frozen semen. Italian frozen semen was brought from Aton Company for Agency and Trade imported from Centro Tori Chiacchierini, Perugia, Italy. The Egyptian one was processed at Theriogenology Department, Faculty of Veterinary Medicine, Benha University from three proven fertile Egyptian breed buffalo bulls aged between 3.5 and 6.5 years old.
Semen evaluation
Straws (n=4/trial/treatment) were evaluated post-thawing at ~37˚C for 30 s. Spermatozoa plasma membrane integrity was evaluated using HOS assay (HOS solution containing0.735 g sodium citrate, 1.351 g fructose and 100 ml distilled water) at 37˚C for 60 min. The percentage of swollen and/or curled spermatozoa were calculated HOS positive (Ramu and Jeyendran, 2013). Eosin-Nigrosin stain was used for sperm morphology evaluation according to the previous report by Andrabi et al. (2008).
Spermatozoa biometry assessment
Images of spermatozoa were chosen and photographed from the stained slides using Euromax microscope (Holland). We used ImageJ software (National Institutes of Health, USA) for the analysis of the biometry parameters analysis. The software was normalized against a decimal scale. Fifty normal spermatozoa were settled and evaluated per slide (n=4 slides/treatment/trial). The units for measurement variables were micrometers (µm) and the ratios were without units. The spermatozoa morphology measured based on the following morphological features:
Head diameter (The length (L), width (W), base width (B)), acrosomal cap length, and width. The head area (µm2), Perimeter (P), Ellipticity (e), Elongation (El), Head shape (HS), Shape factor-1 (Rugosity), Shape factor-2, Shape factor-3 (Regularity) were measured according to Van Duijn (1960) as follows:
Area (A)=1.05-0.081×B2+0.64×W×L
Perimeter=π [3× (L+W)-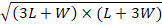
Ellipticity=L/W
Elongation=(L-W) × (L + W)
Head shape=W/L
Shape factor-1 (Sf1) =4πA/P2
Shape factor-2 (Sf2) =Sf1 × (L/W)
Shape factor-3 (Sf3) =[π (L×W)/4]/A
Tail measures: The midpiece width (proximal and distal; µm) and length (µm), principal piece length (µm), flagellum length (µm) and terminal piece length (µm) were measured. The midpiece, principal piece and total flagellum volumes (µm3) were evaluated according to Ros-Santaella et al. (2014).
Midpiece volume (µm3) = 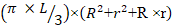
Where L is the midpiece length. R is the half width of proximal midpiece. r is the half width of distal midpiece.
Principal piece volume (µm3) = 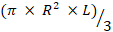
Where R is the half width of midpiece (for example proximal or distal) and L is the length of the flagellum of the principal piece.
Total flagellum volume (µm3) = 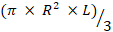
Where R is the half width of midpiece (for example proximal or distal) and L is the length of the flagellum of principle plus the terminal piece.
In vitro fertility assessment
Oocytes collection and selection: The ovaries were collected from the carcasses of buffaloes at Shobra abattoir, brought to the laboratory in warm saline (37°C) supplemented with 100 μg/mL streptomycin sulfate plus 100 IU/mL penicillin within 2 h from slaughtering. After washing the ovaries, oocytes aspirated from mall-to-medium-sized follicles with a 20-gauge needle containing phosphate buffer saline with 3% fetal calf serum (FCS) and antibiotics. Good quality oocytes were selected under the low power of stereomicroscope (20 ×) according to Warriach and Chohan (2004).
Oocytes maturation in vitro: Oocyte maturation step was performed according to the report by Mahmoud et al.(2016a) with modification, 10-20 oocytes were cultured in 100 μl small drop of TCM-199 added with 10% FCS, 50 μg/ml gentamycin sulfate and 50 μM cysteamine covered with mineral oil. Four-well Petri dish was prepared and pre-incubated in a humidified 5% CO2 atmosphere at 38.5ºC for at least 2 h before oocyte insertion, and then incubated for 18-24 h for maturation.
In vitro fertilization and culture:
The fertilization procedure was carried out according to El naby et al. (2017). Frozen straws were thawed at 37ºCfor30 sin a water bath, and then washed by centrifugation (800×g/ 10 min) in bovine serum albumin (BSA)-free Brackett and Oliphant (BO) medium with 10 μg/ml heparin and 2.5 mM caffeine. The sperm pellets were washed with BO medium enclosing 20 mg/ml BSA to adjust the final concentration to 12.5 × 106 sperm/ml. Matured oocytes were washed by BO medium with 10 mg/ml BSA, and then they introduced into 100 μl drops of sperm suspension (10-20 oocytes/drop). Oocytes with spermatozoa were incubated for 5 h in a CO2 incubator. After that, oocytes were washed in TCM-199 to remove loosely attachedsperm. Oocyte groups were again cultured for 6-7 days in previously used maturation media.
In vitro fertility assessment
After the culture period, the developmental competencies of buffalo oocytes were found out. The cleavage, morula, and blastocyst rates were estimated after 2, 5, and 7 days, respectively, for both types of frozen semen.
Statistical analysis
We presented the data as mean ± SE with an independent student t-test using SPSS (Ver. 16). P-value was set at less than 0.05 to define statistical significance.
Results
Semen characteristics and morphometric differences between Egyptian and Italian buffalo bulls
Representative microscopic images of spermatozoa in Egyptian and Italian bulls are presented in Fig. 1. Evaluation of frozen-thawed semen characteristics in Egyptian and Italian buffalo bulls verified substantial differences between the two breeds regarding motility (P<0.001), viability (P<0.001), and abnormalities (P<0.001), in addition to the plasma membrane (P<0.001) and acrosome integrities (P<0.01) (Table 1).
Table 1: Frozen-thawed semen characteristics in Egyptian and Italian buffalo bulls
| Parameter | Egyptian bulls | Italian buffalo bulls |
P value (t-test) |
| Motility | 43.00±0.82 |
61.00±1.00*** |
< 0.001 |
| Viability | 60.90±1.08 |
73.00±0.98*** |
< 0.001 |
| Abnormality |
15.30±0.47*** |
11.40±0.45 | < 0.001 |
| Acrosome integrity | 77.30±1.25 |
83.00±0.95** |
< 0.01 |
| Plasma membrane integrity | 73.20±0.98 |
80.80±1.04*** |
< 0.001 |
Data are presented as Mean ±SE (n=4). **= P< 0.01. ***= P<0.001
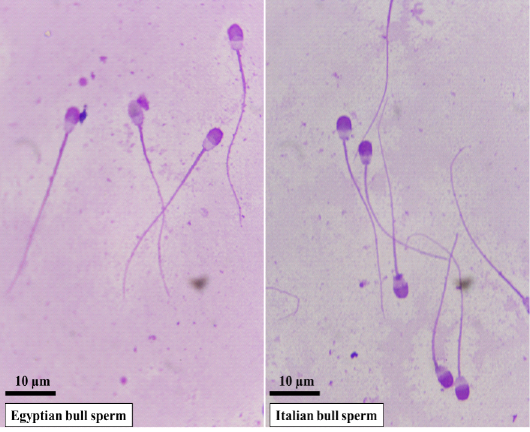
Figure 1: Representative microscopic images of spermatozoa in Egyptian and Italian buffalo bulls stained with Giemsa solution and examined under oil immersion lens (×100). The sperm head size and shape differ in Egyptian buffalo bull versus Italian bull with longer and pyriform shape.
Table 2: Sperm head biometry in Egyptian and Italian buffalo bulls
| Parameter | Egyptian bulls | Italian buffalo bulls |
P value (t-test) |
|
Head length (μm) |
8.066±0.104*** | 7.230±0.058 | < 0.001 |
|
Head width (μm) |
4.557±0.077 | 4.623±0.038 | 0.411 |
|
Head base (μm) |
2.028±0.073 | 2.230±0.054* | < 0.05 |
|
Acrosome length (μm) |
4.633±0.169 | 4.337±0.066 | 0.090 |
|
Acrosome width (μm) |
4.542±0.063 | 4.685±0.029* | < 0.05 |
|
Head area (μm2) |
29.206±0.590** | 27.174±0.337 | 0.002 |
|
Head perimeter (μm) |
32.343±2.412 | 37.679±0.246* | < 0.05 |
| Rugosity | 0.225±0.002 | 0.240±0.001*** | < 0.001 |
| Ellipticity | 1.781±0.033*** | 1.568±0.016 | < 0.001 |
| Elongation | 0.297±0.021*** | 0.220±0.005 | < 0.001 |
| Head shape | 0.552±0.018 | 0.641±0.007*** | < 0.001 |
| Shape factor-1 | 0.225±0.002 | 0.240±0.001*** | < 0.001 |
|
Shape factor-2 |
0.399±0.006*** | 0.376±0.003 | < 0.001 |
| Shape factor-3 | 0.986±0.007** | 0.966±0.002 |
< 0.01 |
Data are presented as Mean ±SE (n=4). *= P<0.05. **= P<0.01. ***= P<0.001.
Table 3: Sperm tail biometry in Egyptian and Italian buffalo bulls
| Parameter | Egyptian bulls | Italian buffalo bulls |
P value (t-test) |
| Midpiece width, proximal (µm) | 2.962±0.462*** | 0.876±0.028 | < 0.001 |
| Midpiece width, distal (µm) | 1.931±0.248*** | 0.633±0.023 | < 0.001 |
| Midpiece length (µm) | 11.246±0.231 | 10.964±0.301 | 0.473 |
| Principal piece length (µm) | 38.705±0.812 | 40.945±0.469** | < 0.01 |
| Terminal piece length (µm) | 4.730±0.319 | 4.385±0.166 | 0.297 |
| Flagellum length (µm) | 43.202±0.723 | 56.294±0.591*** | < 0.001 |
| Midpiece volume (µm) | 17.242±1.566*** | 10.083±0.65566 | < 0.001 |
|
Principal piece volume (µm3) |
14.016±1.270*** | 8.663±0.561 | < 0.001 |
|
Total flagellum volume (µm3) |
15.638±1.412* | 11.948±0.793 |
< 0.05 |
Data are presented as Mean ±SE (n=4). *= P<0.05. **= P<0.01. ***= P<0.001.
Table 4: Developmental competence of buffalo oocytes fertilized by Egyptian and Italian semen
| Parameter | Egyptian bulls | Italian buffalo bulls |
P value (t-test) |
|
| Inseminated oocytes | n | 94 |
92 |
|
| Cleaved oocytes | n | 59 | 66 |
ns |
| % | 62.40±2.50 | 69.84±3.30 |
ns |
|
| Morulae | n | 36 | 40 |
ns |
| % | 38.90±1.60 | 42.90±2.10 |
ns |
|
| Blastocysts | n | 9 | 17 |
ns |
| % | 9.40±1.00 | 17.80±2.00* |
< 0.05 |
Percent (Mean ±SE, n= 3 replicates) from total fertilized oocytes.n: The overall number in all replicates. * P<0.05 (Student t-test)
Data of sperm head biometry in Egyptian and Italian bulls are shown in Table 2. Spermatozoa of Egyptian bulls were characterized by longer head length, larger head area, and higher ellipticity. Italian buffalo spermatozoa were characterized by a wider head base and acrosomal width, larger head perimeter, higher rugosity, and difference in head shape.
Assessment of sperm tail biometry in Egyptian and Italian buffalo bulls showed marked differences between the two breeds (Table 3). The total flagellum and principal piece length were longer in Italian bulls than that of Egyptian bulls. Midpiece (volume and width) and principal piece (volume) measures were higher in Egyptian bulls compared with the Italian bulls.
In vitro fertility indices in Egyptian and Italian buffalo bulls
Data presented in Table 4 showed the developmental competence of buffalo oocytes after fertilization by Egyptian and Italian semen. There were no statistically significant differences in the cleavage and morula rates between the Egyptian and Italian semen. However, blastocyst rate was significantly (P<0.05) higher in Italian than that shown in the Egyptian semen.
Discussion
Computer-assisted sperm morphometry is a precise method for assaying sperm biometry in buffalo bulls. Semen pictures and sperm morphometry can be valuable tools for developing a fertility index. Various factors are involved in semen quality including breed, age, method of collection and environmental conditions. In the current study, Egyptian and Italian breeds showed differences in sperm quality, biometry, and fertilizing capacity.
Bull selection is the master target to achieve higher fertility rates among buffalo bulls (Abdel Dayem et al., 2009), and this is governed by different sperm components that responsible for male fertility and phenotypic differences (Fitzpatrick and Lupold, 2014). The biometry indices for buffalo spermatozoa in our study were in accordance with former studies in Egyptian (Kandiel et al., 2017) and Italian (Serafini et al., 2016) breeds. However, as toour best knowledge, no previous report compared the dimensions of Egyptian and Italian buffalo spermatozoa. In our study, evaluation of frozen-thawed semen affirmed the presence of substantial differences between the two breeds regarding semen characteristics (motility, viability, abnormalities, and plasma, and acrosomal membrane integrities),sperm head (head length, perimeter, area, acrosomal width, and shape), and sperm tail (midpiece, principal piece, and total flagellum) biometry. Spermatozoa are subjected to selection after their deposition in the female tract. However, the biophysical relation between biometry and motility may impact the advancement of spermatozoa through the female reproductive system (Garcia-Vazquez et al., 2016). The differences in the biometry between buffalo/cattle bull breeds have been declared in previous works (Aggarwal et al., 2007; Koonjaenaket al., 2007). The insignificant variation in mid-piece in this study agreed with former findings by Shahani et al. (2010).The variation in sperm length is principally related to the differences in tail length. The longer the flagellum of spermatozoa, the greater the forces generated due to its motion and requirement for immediately available energy (Katz and Drobnis,1990). There is a relationship between morphology (flagellar length), flagellar beat frequency, and the swimming speed (Dresdner and Katz, 1981). Former studies indicated the value of motility and viability rates as a predictive measure in semen evaluation (Mahmoud et al., 2013), and that the acrosome integrity percentages and pregnancy rate in buffalo were significantly lower in Egyptian than Egyptian-Italian crossbred bulls (Mahmoud et al., 2016b).
The quality of frozen semen is an emphatic signal for the in vitro fertility rate (Graham and Moce, 2005).In our study, the cleavage and morula rates did not vary between oocytes fertilized by Egyptian and Italian semen. However, the blastocyst rates were markedly lower in oocytes fertilized by Egyptian semen than those fertilized by Italian semen. As to our best knowledge, scarce issues distinguished between the fertilizing ability of Egyptian and Italian frozen semen in vitro (Soliman et al.,2018). It was known that there was a link between field non-return rates and IVF (Lonergan,1994). Mahmoud et al. (2016b) showed that the cross-breeding between Italian and Egyptian breeds improved the integrity of acrosome and pregnancy rates over Egyptian bulls. Pregnancy rates in Egyptian buffaloes ranged from 41.2-47.4% (Mahmoud et al., 2013). In conflict, Anzar et al.(2003) proved that the fertility rate of Nili Ravi buffalo’s semen was 33%, whereas the pregnancy rate was 45.2% with Italian buffalos’ semen (Barile et al.,1999). On the other hand, in cattle bulls, Al Naib et al. (2011) stated that high fertile semen is more effective in penetrating artificial mucus and oocytes in vitro and ratio of pregnancy to 50% in vivo. Thus, the variances in pregnancy and blastocyst rates were associated with the sperm cell fertilizing ability to oocytes.
Conclusion
Our study findings revealed for the first time the significant variations in sperm biometry between Egyptian and Italian semen/spermatozoa and this could afford an explanation on the differences in the in vitro fertility potentials between the two breeds, and the resulting higher IVF outcome for Italian semen.
acknowledgements
This research was supported by National Research Centre, Cairo, Egypt. Project number 1102101.
Conflicts of Interest
The authors declare no conflict of interest.
authors contribution
Al.Al. H.El., M. M. M. K., K. Gh. M. M.: designed and performed the experiments and also wrote the manuscript. G. A. El., W. T. M. S. and Al.Al. H.El.: performed, semen analysis and in vitro fertilization. Y. F.A.: completed data analysis, revision, and writing of the article. All authors read and approved the final manuscript.
References






