Advances in Animal and Veterinary Sciences
Research Article
Circulating Redox Status in the Bedouin Egyptian Oases Camels (Camelus dromedaries) During the Peripartum Period
Mostafa A. Saleh1, M. H. Rateb2, Gaadee H.I.M2*, Nasser S. Abou-Khalil3, Mervat S. Hassan4
1Biochemistry Unit, New Valley Animal Health Research Laboratory, Animal Health Research Institute, Agriculture Research Center, New Valley, 725211, Egypt; 2Biochemistry Unit, Assiut Animal Health Research Laboratory, Animal Health Research Institute, Agriculture Research Center, Assiut, Egypt; 3Department of Medical Physiology, Faculty of Medicine, Assiut University, Assiut, 71526, Egypt; 4Department of Theriogenology, Faculty of Veterinary Medicine, The New Valley University, New Valley, 725211, Egypt.
Abstract | The camel forms an integral part of the culture and agriculture of many countries and has done so for thousands of years. How it integrates varies greatly from; the supremerely sophisticated racing camel in the Arabian Gulf. This study aimed to explore the oxidative stress index (OSI, a ratio between pro-oxidants and antioxidants) in the blood serum of camels (Camelus dromedaries) during the peripartum period. Sera samples were collected from ten late pregnant and ten non-pregnant, non-lactating (as a baseline status, BL) multiparous nomadic Balady camels in their natural habitat in the Egyptian oases concerning sampling weekly throughout the last three weeks prepartum, day of calving and weekly throughout the first three weeks postpartum. Total oxidant status (TOS) and total oxidant capacity (TAC) did not differ between pre and postpartum periods and they were within the values of the basal non pregnant- non lactating (NPNL) state. At parturition the values were higher (P < 0.05) than the peripartum and the BL concentrations. Accordingly, the calculated OSI values showed a roughly stable trend (P > 0.05) and did not achieve a clear tendency or statistical significance among sampling times. A positive correlation of TOS with oxidant stress index (0SI) (P<0.001) and TAC (P < 0.001) was noticed. From a redox standpoint, the stability of OSI indicates that the redox system is adapted to the homeostatic status during the peripartum period in nomadic camels. This may indicate that, OSI is not a challenge of these peripartum camels, where the generated redox oxidative stress (ROS) is attacked by a superior defense potential to neutralize it. This study may aid in a better understanding of the physiological adaptation mechanisms of camels and may be useful for comparative studies with other species.
Keywords | Oxidative stress index, Peripartum, Oasis, Egyptian camel
Received | April 08, 2021; Accepted | April 30, 2021; Published | July 28, 2021
*Correspondence | Gaadee HIM, Biochemistry Unit, Assiut Animal Health Research Laboratory, Animal Health Research Institute, Agriculture Research Center, Assiut, Egypt; Email: [email protected]
Citation | Saleh MA, Rateb MH, Gaadee HIM, Abou-Khalil NS, Hassan MS (2021). Circulating redox status in the bedouin egyptian oases camels (camelus dromedaries) during the peripartum period. Adv. Anim. Vet. Sci. 9(9): 1390-1395.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1390.1395
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Gaadee et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Camels bear exceptional genotypes and phenotypes that are different from any other well-being for metabolic processes and adaptation to the harsh environment (Faye and Bengoumi, 2018; Tibary and El Allali, 2020). Interestingly, the camel employs brilliant immunological and molecular mechanisms against pathogenic agents and pathological conditions. The immune system consists of a complex network of cellular and non-cellular components, which contribute equally to an effective immune response against pathogens (Ali et al., 2019). Recently, the importance of camel breeding in Egypt has increased; however, one of the major limitations in this industry is the extremely low reproductive efficiency (El-Bahrawy et al., 2015). Where during the transition from late pregnancy to early lactation (the peripartum period), ruminants may fail to adapt to the profound increase in nutrient requirements associated with fetal growth, parturition and lactogenesis, and develop metabolic stress (Sordillo and Mavangira., 2014; Abuelo et al., 2019). Camels have a unique ability to control their lipolytic and gluconeogenic rates, under diverse conditions of increased metabolic demands (Faye and Bengoumi, 2018). However, the peripartum period in camels is characterized by acute inflammatory processes and fluctuations in metabolic and hormonal homeostasis (Tharwat et al., 2015; Tharwat and Al-Sobayil, 2015; Ahmadpour et al., 2019). These alterations in the physiological conditions trigger neutrophils, macrophages and enhance the generation of pro-inflammatory cytokines such as interleukin 1 (IL-1), IL-6, tumor necrosis factor-α (TNF-α) and other chemotactic factors such as IL-8 (Celi and Gabai, 2015), and provoke the generation of free radicals and pro-oxidants (Abuelo, et al., 2019).
Total oxidant status (TOS) has been used for several years as an indirect biomarker for the enhanced oxidation state in the body, reactive oxygen species (ROS), and oxidative stress Fereidoon and Ying (2015). Besides the superoxide anion (O̅֗2), ROS includes the peroxide functional group (ROOR′), which is considered as structural derivatives of H2O2 (Halliwell and Gutteridege, 2015). These metabolites exert crucial effects on cell disruption, cytotoxicity, DNA damage and pathological processes during the transition period in ruminants (Abd Ellah, et al., 2016; Celi and Gabai, 2015). As a defense, the body has a sufficient enzymatic and non-enzymatic antioxidant array that acts in a harmony and fine-tuned mechanism to neutralize the continuous production of oxidants during metabolism (Carocho and Ferreira, 2013). The measurement of the total antioxidant capacity (TAC) considers the cumulative action of all antioxidants and provides relevant information that may effectively describe the dynamic equilibrium between pro-oxidants and antioxidants Fereidoon, and Ying (2015). Oxidative stress (OS) arises when oxidant substances overwhelm antioxidant defenses; it may therefore cause an increase in the generation of oxidation by-products and a decrease of antioxidant protection mechanisms (Sies et al, 2017).Redox balance plays a key role in ensuring a satisfactory preipartum period in ruminants; however, OS resulted from redox imbalance has been linked to peripartum diseases (Abuelo et al., 2019). Enhanced TOS, decreased TAC and evoked OS was demonstrated in several studies in peripartum cows (Abd Ellah, 2010, 2016; Abuelo et al., 2019), sheep (Mutinati, 2013) and goats (Radin et al., 2015). The TOS to TAC ratio was regarded as a novel tool to measure oxidative stress index (OSI) that could be used as an approach to study redox homeostasis in veterinary medicine (Abuelo et al., 2013) and determine which factors have the greatest influence on oxidative status (Urh et al., 2019) during the peripartum period in cattle.
To the best of our knowledge, to date, there is no scientific report on estimating the redox status in the peripartum camels. The present study aimed to measure the redox homeostatic status and deducing the OSI in the nomadic camel by using the ratio between TOS and TAC during the peripartum period.
MATERIALS AND METHODS
Study area
This study was carried out in El-Kharga oasis, El Wadi El-Gadid Province (in the Western Egyptian Desert) during September 2020.El-Kharga oasis is a depression that lies between 22° 30′ and 26° 00′ N latitudes and between 30° 27′ and 30° 47′ E longitudes. The climate of this area is arid and rainfall is almost absent. During the study trial, the mean atmospheric temperature and relative humidity in this oasis were 36.5 ± 2.5°C and 31 ± 4%, respectively.
Animals
The nature of camels in this oasis is nomadic. They browse freely on the scattering ephemeral vegetation and bushes, mainly German grass (Haloxylon salicornicum), Kassala (Cyperus conglomerates), and sometimes Barseem (Medicago sativa) is offered without concentrate or mineral supplementations. The chance of watering depends on water pumped from the few scattering ground wells or water passages when available. Water quality assessment by water research institute, includes an evaluation of the physical, biological and chemical properties of water in relation to the natural quality and the results revealed the water fit for the consummation.
Ten pregnant and ten non-pregnant non-lactating (NPNL) multiparous Balady camels were selected and enrolled in this study. They were selected randomly and depending on the balance of the expected calving date in consultation with owners or experienced camel’s breeders who had known their mating history. The mean body weight of these camels ranged from 300-350 kg. Their mean age was 13.2 years (11-15) and their body condition score ranged from 2.5 to 3.0 (scales 0-5); Iglesias et al (2020). All the selected camels have been reared similarly in their natural habitat in the desert environment under unorganized farming with unsatisfactory standards of animal management and feeding (nomadic system). These animals were identified by their fire tattoo. According to clinical examination (Temperature, Pulse, Respiratory rate and body condition) and laboratory investigations (Direct examination by microscopically and indirect examination by serological), these camels were clinically healthy, free from parasites and systemic diseases.
The pregnant camels were balanced across the expected calving date and were observed throughout the last three weeks of pregnancy, the day of parturition (within 12 hours) and the first three weeks after calving. All pregnant camels had normal easy calving without clinical abnormalities. The NPNL camels were established as a baseline (BL) control group, when neither lactation nor pregnancy was major metabolic burdens (Seri et al., 2015). Their biochemical values were compared with those obtained from the transitional camels.
Blood sampling
Blood samples were collected from all the selected she-camels before the morning feeding by the jugular vein puncture in a 10-ml vacuumed tube without anticoagulant. From the pregnant camels, blood was collected weekly throughout the last three weeks prepartum (PrP), at calving time (within 8 h) and weekly throughout the first three weeks postpartum (PsP). The accurate estimation of the weeks of PrP sampling was assessed concerning the calving time. Samples were immediately sent to the laboratory on crushed ice for subsequent sera collected by centrifugation at 1500 ×g for 15 min at 4 °C. The collected clear, non-hemolyzed sera were frozen at -20°C until analysis.
Biochemical analysis
The biochemical analysis of sera samples included total oxidant status (TOS) were measured by using test kits (Spectrum, diagnostic, Cairo. Egypt) according to manufacture instruction UV/VIS spectrophotometry calorimetrically at 560 nm, and expressed in µmol H2O2 Equiv/L ,according to Fereidoon, and Ying, (2015);
Total antioxidant capacity (TAC) was determined after the method described by Yang et al. (2017), by using the colometric ABTS assay kits (Beyotime institute of Biotechnological, Haimen, China) and expressed as mmol Trolox equiv /l..The TAC unit, mmol Trolox equivalent /L, was converted to µmol Trolox equivalent/L. The OSI value was calculated as follows: OSI (Arbitrary unit) = (TOS, µmol/L / TAC, µmol Trolox equivalent /L) ×100 (Dikalov and Harrison, 2014).
Statistical analysis
Statistical procedures were carried out using the packaged SPSS programmer for windows version 20.0.1 (SPPS, Chicago, 11, USA). The normal distribution of data of each parameters was checked using the Shapiro-wilk test.Data were analysed by using an analysis of variance( one-way ANOVA).repeated measure procedure followed by means of pairs multiple comparison procedures using Duncan’s new multiple range test to determine the differences between sampling times for each parameters. Data was expressed as mean ± standard error (SE). Differences were considered significant at P < 0.05. Statistical results expressed in (Table 1).
Table 1: mean values ( se) of total oxidant status (TOS), total antioxidant capacity (TAC) and oxidative stress index (OSI) in non-pregnant non-lactating (NPNL), prepartum (PrP), parturient (part) and postparturent (PrP) camels.
| NPNL | PrP | Part | PsP | |
|
TOS (µmol H2O2 Equiv/L) |
10.22 1.55 |
12.04 1.01 |
16.72* 1.33 |
12.71 1.43 |
|
TAC (mmol Trolox equiv/l) |
0.784 0.09 |
0.895 0.04 |
1.152* 0.11 |
0.945 0.05 |
| OSI (Arbitrary unit) |
0.013 0.0009 |
0.0135 0.0008 |
0.0145 0.0011 |
0.0135 0.0008 |
* Significantly different from NPNL at P < 0.05
RESULTS
The results illustrated in Table 1, where oxidative status markers including TOS, TAC and OSI in the non-pregnant, non-lactating, peripartum, parturient, and postpartum camels. The Value of the NONL was considered as a baseline. The cumulative TOS, TAC and OSI values did not differ during the peripartum period, but the values of the TOS and TAC increased compared with the BL value.
In comparing the values during the different sampling times, the TOS (Fig. 1a) as a representative of ROS, showed unvarying values (P > 0.05) during the early PrP weeks (Weeks -3 and -2) and when compared with the BL data. However, the value began to increase significantly (P < 0.05) at the first week just before calving compared with BL data. At parturition, the TOS peaked to reach its maximal level, where the value was significantly (P < 0.05) higher than the PrP and the BL concentrations. After calving, the value decreased gradually (P < 0.05) until reaching the levels of PrP and the BL values in the second and third weeks PsP.
Levels of TAC (Fig. 1b) showed a relatively steady state (P > 0.05) throughout the PrP stage and did not differ significantly from the BL value. At parturition there was a remarkable increase (P < 0.05) in TAC compared with the W-3 and BL values. After calving, the value returned to reach the PrP and BL level. Accordingly, the calculated OSI (Fig. 1c) values showed a roughly stable trend (P > 0.05) and did not achieve a clear tendency or statistical significance (P > 0.05) among sampling times.
Pearson’s correlation (r) and linear regression analysis of the paired data obtained by the individual cases (Fig. 2), revealed that TOS was positively correlated with TAC (r = - 0.385, P < 0.001).
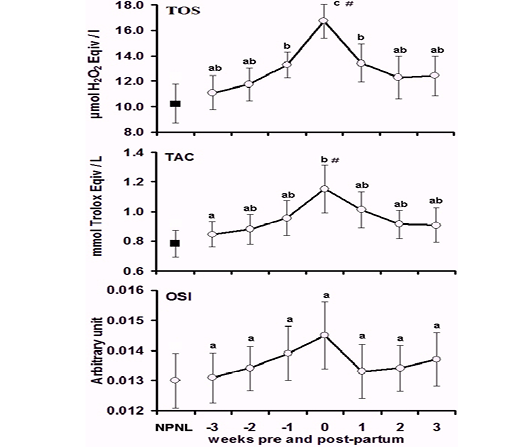
Figure 1: Concentrations mean (SE) of total oxidant status (TOS, µmol/L), total antioxidant capacity (TAC, µmol Trolox equv/L) and oxidative stress index (OSI, Arbitrary unit) in the non-pregnant non-lactating (NPNL) and peripartum Bedouin camels (N=10/period). a,b,c Values with unlike descriptive superscript letters are significantly different at P < 0.05 within the peripartum period # Values are significantly different from the basal NPNL at P < 0.05
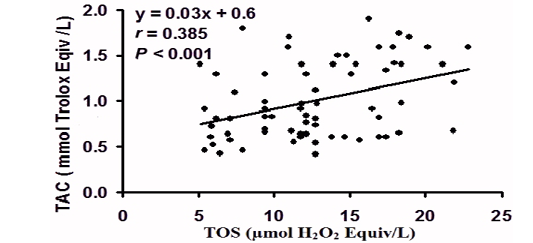
Figure 2: Pearson’s correlation (r) and linear regression analysis of the paired data obtained by the individual cases of total oxidant status (TOS) with total antioxidant capacity (TAC) ) in peripartum Bedouin camels (N=70).
Unlike the transition period in other ruminants, the periparturient period in dromedary camels has gained very little attention. It is worth saying that this is the first report that describes redox homeostatic status in the peripartum camel. In this study, we observed an increase in the TOS and TAC at parturition in the dromedary camel, whereas the OSI was stable throughout the peripartum period in a way suggesting adaptation toward redox homeostasis.
Discussion
Levels of ROS increased gradually throughout the peripartum period in cattle and goats (Celi, et al., 2010; Seo et al., 2014). Our results show stability of ROS concentrations with a transitory significant increase only at the time of calving. This suggests the superiority of camels in controlling extra free radical production during the peripartum period, except in time of parturition, indicating a temporary imbalance between ROS production and the neutralizing capacity of antioxidant mechanisms at this period. Undeniably, parturition is an inflammatory process characterized by augmented endocrine and metabolic activity, sharp physical effort, deep structural and functional reorganizations of the uterus and cervix to expel the fetus and placenta (Nagel et al., 2019). These changes enhance the massive influx of triggered neutrophils and monocytes with a subsequent dramatic explosion of free radicals and the production of ROS (Halliwell and Gutteridege, 2015). Further, cytokines activate the pro-inflammatory transcription factor, nuclear factor-kappa B (NF-κB) which further amplifies free radicals and ROS production (Sies et al., 2017).
Total antioxidant capacity is an analyst frequently used to assess the antioxidant status of biological samples and can evaluate the antioxidant response against the free radicals produced in a given disease. Fereidoon and Ying, (2015); during the peripartum period, a depletion of TAC to combat the increase in ROS generation was reported in camel (Hawida et al., 2017). Our results revealed enhanced TAC at parturition, suggesting an augmented antioxidant potential to cope with the amplified ROM at this time. It was reported that enzymatic antioxidants in camels have unique molecular, biochemical and superior defense potential compared to other mammals; for instance, camel SOD proceeds faster, preventing the accumulation of OS generated (Al-Otaiba et al., 2010; Faye and Bengoumi 2018; Chafik et al., 2019). The strong positive correlation between TOS and TAC in our camels confirms these results and suggests that the entire TOS and TAC appear to be under homeostatic control.
It is difficult to determine whether and when animals are experiencing OS. Therefore, in veterinary medicine the ‘ideal’ biomarker does not exist and a panel of measurements is crucial to dutifully evaluate OS changes (Abuelo et al., 2013). Oxidative stress is an imbalance toward the pro-oxidant side of the pro-oxidant/antioxidant homeostasis, so that OS can result from increased oxidants, decreased antioxidants or even both (Halliwell and Gutteridege., 2015). This means that the players in redox homeostasis are TOS and TAC (Valacchi et al., 2018). OSI provides an objective judgment of the relationship between oxidants and antioxidants, not seen by the separate determination of both components (Abuelo et al., 2013). In cattle, OSI was higher PsP than those before parturition indicating animal’s risk to develop postpartum diseases (Abuelo et al., 2013; Urh et al., 2019). The absence of variations of OSI between periods in our study, even at parturition, suggests that the high burden of ROS does not mean a high OS, which could be governed by an efficient TAC. Our results confirm those obtained by (Urh et al., 2019) who gave an emphasis to the value of exploring both sides of the TOS and TAC balance because greater concentrations of TOS might not always result in greater OS but might be controlled by a greater capacity of antioxidants. Desert camels exhibit appreciable metabolic adaptation and redox defense mechanisms against ROS and oxidative stress. (Al-Otaiba et al., 2010) suggested the existence of mechanisms of protection in camel tissues against the deleterious effects of ROS and oxidative stress. Camel tissues have a significant ability to metabolize ROS in comparison with other animals. Camel cytochrome enzymes are specially developed and adapted to the camel environment. As a form of adaptation, the camel mitochondrial proteins showed a higher evolutionary rate in camels to allow for living in different environments (Altaher et al., 2015).
CONCLUSION
From a redox standpoint, the stability of OSI indicates that the redox system is adapted to the homeostatic status during the PP in nomadic camels. This may indicate that, OS is not a challenge of for these peripartum camels, where the generated pro-oxidants are attacked by a superior defense potential that can neutralize it. This study may aid in a better understanding of the physiological adaptation mechanisms of Bedouin camels and may be useful for comparative studies between camels and other species.
Conflict of interest
The authors have no conflicts of interest to declare. None of the authors of this paper have a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Ethics approval
This study was approved by the ethical rules of the Animal Health Research institute. Agriculture research center.Egypt, and in accordance with local laws and regulation (NO.17101)
Authors’ contribution
The authors participated and contributed sufficiently and equally in the completion of the manuscript, developed The hyposis, performed the computations, verified the biochemical studies. Performed analysis, interpret the results, and contribute to the final manuscript.
REFERENCES






