Advances in Animal and Veterinary Sciences
Research Article
Improving the Reproductive Performance of Cows Suffering from Ovarian Hypofunction using Herbal Supplement
Lukman Affandhy*, Muchamad Luthfi, Frediansyah Firdaus, Dian Ratnawati, Risa Antari
Beef Cattle Research Institute, Indonesia.
Abstract | This study aimed to solve the reproductive problems in crossbred cows suffering from ovarian hypofunction (HPO). Twenty-four cows were divided into four treatments with six replications, treatment A concentrate feed (control); treatment B: control + premix; treatment C control + two boluses, and treatment D control + four boluses. The experimental period was 42 days (two estrous cycles). The bolus formulation consisted of vitamin A, vitamin D, vitamin E, zinc, Moringa oleifera and alginate powder. Data analysis using oneway ANOVA with a Completely Randomized Design. The results showed that the lowest number of follicular palpations in cows suffered from HPO was 67% compared to 83% premix administration, while all cows given bolus were obtained follicular palpation or cured 100%. The results showed that the follicle diameters of the four treatments were not significant difference, but the results of rectal palpation were almost palpable, except treatment A on day 21 that could not be palpated on the left follicle and no corpus luteum was found, whereas in treatments B, C and D partially luteal phase were found. It was concluded that the best treatment of HPO in cows suffered from HPO was by providing two boluses, and was administered twice a week.
Keywords | Cows, Herbal supplement, Follicle, Corpus luteum
Received | January 27, 2021; Accepted | February 08, 2021; Published | May 25, 2021
*Correspondence | Lukman Affandhy, Beef Cattle Research Institute, Indonesia; Email: [email protected]
Citation | Affandhy L, Lufti M, Firdaus F, Ratnawati D, Antari R (2021). Improving the reproductive performance of cows suffering from ovarian hypofunction using herbal supplement. Adv. Anim. Vet. Sci. 9(6): 862-868.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.6.862.868
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Affandhy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Reproductive disorders are a major problem in reducing reproductive efficiency and resulting in decreased cow productivity (Abdisa, 2018). One of the causes is the impact of nutrition on the reproductive performance of cows, several studies have shown that the effect of nutrition is created through a complex interaction of various factors including content and use of body reserves, distribution of nutrients between different systems and organs and the use of nutrients for various functions of related aspects with the reproductive performance (Izquierdo, 2016), including negative energy balance that is an important factor in ovarian inactivation and affects the health and reproduction of cows. (Fan et al., 2017). Ovarian hypofunction is a condition where no follicle growth and corpus luteum. This is caused by the decline of the concentration of Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH) in the circulation when cows experience prolonged nutritional deficiency. Budiyanto et al. (2016) reported that 57.95 % of Bali cows suffered from reproduction problems such as delayed puberty, ovarian hypofunction, metritis, endometritis, and prolonged anestrus postpartum period. The interaction between some factors such as environment or management, individual responses, type of reproduction problem, and the severity of reproductive issues will show the different responses to the treatments. Reproductive disorders in cows on farm include follicular cysts 18 days after artificial insemination, was 52.2% ovarian hypofunction and 6.6% endometritis (Oktarianti, 2017). The types of ovarian disorders that were detected were hypofunction (19.32 %), cystic follicles (8.21 %), corpus luteum persistent (4.5 %), atrophy (1.93 %), and partial agenesis (0.97 %) (Rosadi et al., 2018). The reproduction issues in cows kept by smallholder farmers were ovarian hypofunction (6.25 %) (Sutiyono et al., 2017). While Azharuddin et al. (2017) stated that the reproductive issues in cows kept by smallholder farmers in Lamongan district was about 340 heads and most of them (40 %) suffered from HPO.
A study reported that the most efficient cows were mated on 60-90 days after calving, with anestrus post partum was 71,5±15,9 days and the conception rate was 64,91% (Affandhy et al., 2019). A success study reported that treatments with vitamin A, D, and E injection, feeding premix, and anthelmintic cured the HPO in Ongole crossbred, Limousin, and Simmental cows; based on the morphological and normal functional follicles, 72.9% of them recovered (Widarini et al., 2017). While Randia dumetorum and Tinospora cordifolia combination had an effective role in the treatment of anoestrus buffalo heifers (Chaudhiry et al., 2018).
Recently, the treatments for ovarian hypofunction are not practical due to the techniques such as injection, feeding, and anthelmintic treatment. Therefore, the more practical treatments are needed through vitamin-mineral in bolus supplementation that can be administered orally for HPO treatments.
Ovarian hypofunction treatments have used hormones that were expensive and were mostly imported, such as GnRH (Suartini et al., 2013; Afriani et al., 2014); FSH and LH (Raju et al., 2013); Leptin (Laksmi et al., 2016). Vitamin D altered the differentiation and phenotypic of the white adipose tissues where leptin modulated lipid and energy metabolisms (Narvaez et al., 2018). Khatti et al. (2017) reported that antioxidant supplementation and the increase of energy level by increasing 20 % of the concentrate diet, vitamin E (80 IU/kg DM/day), and selenium (0.3 mg/kg DM/day) provided glucogenic nutrition for positive energy balance that could maintain the concentrations of insulin, insulin-like growth factor-1 (IGF-1), and leptin. Thus, early ovulation occurred after parturition. One point five mg/ml of sodium selenite injection and 50 mg/ml of vitamin E increased LH of Fries Holstein cows on days 25 and 45 post partum (Prasetiani et al., 2015). Laksmi et al. (2016) reported that the injection of 100-200 µg/head of leptin to Bali cows increased the concentration of FSH that contributed to the growth of follicles in the ovarium.
This research aimed to solve the ovarian hypofunction in crossbred (limousin x Ongole crossbred) cows through multivitamin-minerals and herbal supplementation.
MATERIALS AND METHODS
The following experiment was conducted under the guideline of the Indonesian code of Practice for the care and use of Animals for Scientific Purposes and was approved by the Indonesian Ministry of Agriculture Animal Ethics Commite (Balitbangtan/Lolitsapi/Rm/07/2019).
Time and Location of Study
This study was carried out at the farmer group Niky Mapan, Sumbersuko District – Lumajang Regency (East Java) that suffered from HPO (one time calving and body condition score < 2) for three month, the bolus making was done at The Indonesian Beef Cattle Research Station (IBCRS) Laboratory. Hormonal analysis was performed at the Institute of Tropical Disease, Faculty of Veterinary Medicine, The University of Airlangga.
Research Materials
The current experiment was carried out on farm to heal HPO using vitamin, mineral, and herbal supplementation in the boluses for 24 cows that were allocated into one of four different treatments. The boluses were made from a half dosage of NRC (2000), containing vitamin A (retinol) 1400 IU/kg DM (0,3 µg/ IU), vitamin D3 (cholecalciferol) 137.5 IU/kg DM (0.025 µg/IU), vitamin E (tocopherol) 30 IU/kg DM (1 mg/IU), zinc 30 mg/kg DM, Moringa oleifera 125 mg/kg liveweight (Amelia et al., 2018) and alginate was used as an adhesive (30 % of the weight of all boluses that were made). The treatments were A: concentrate diet (control), B: concentrate diet + premix, C: concentrate diet + two boluses D: concentrate diet + four boluses. The supplements were given twice a week.
Sample Collection
Before the experimental period, all cows underwent induction such as treatment with the endoparasite broad-spectrum (anthelmintic). Twenty four cows were selected from the farmer group Niky Mapan, Sumbersuko District – Lumajang Regency (East Java) that suffered from HPO (at the first calving time and body condition score < 2); with six replications in each treatment. The cows were housed in the individual pens. The experimental period was 42 days (about two estrus cycles), in every cycle, ovarium was observed to check the development of follicles, the size, and shape of the ovarium using rectal palpation and ultrasound. The concentration of FSH and LH have observed in the blood samples that were taken through a jugular vein using a vacuum tube without coagulant, then was centrifuged at 6.000 rpm in the Reproduction laboratory at the IBCRS. The serums then were placed in Eppendorf and were frozen at ≤ 20°C; for hormonal analysis. The blood samples were taken at t1 (day 0), t2 (day 21) and t3 (day 42) in each treatment at 06.00 – 08.00 AM.
Table 1: Chemical composition of the feed ingredients fed to cows in the current experiment
|
Feed |
Dry matter | Crude protein | Extract ether | Crude fiber | Ash | TDN* |
|
% |
||||||
| Concentrate diet | 77.17 | 9.57 | 6.78 | 23.69 | 18.58 | 65.00 |
| Elephant grass | 21.00 | 8.30 | 3.32 | 29.39 | 8.64 | 50.00 |
| Coffee shell | 86.93 | 12.50 | 0.40 | 37.87 | 16.34 | 50.60 |
| Rice brand | 90.90 | 11.97 | 5.29 | 23.26 | 19.23 | 39.00 |
| Rice straw | 82.10 | 3.70 | 1.37 | 45.23 | 21.26 | 46.65 |
* The data was analyzed from the proximate analysis results carried out by Nutrition and Feed laboratory at the Indonesian Beef Cattle Research Station.
Table 2: The concentration of FSH in the cows administered premix for ovarian hypofunction treatment.
| Treatments | T1 | T2 | T3 |
|
ng/ml |
|||
| A | 3.78 ± 1.95 | 3.59 ±2.40 | 3.03 ±2.15 |
| B | 5.03 ± 3.77 | 4.98± 3.71 | 2.77± 2.61 |
| C | 6.77± 6.66 | 6.92± 6.17 | 3.32± 3.61 |
| D | 3.84± 2.93 | 2.62± 2.02 | 2.13± 1.19 |
Description: A without bolus (control), B Premix, C two boluses, and D four boluses (P>0,05). T1=day 0, T2=day 21 and T3=day 42 after treatment .
Feeding Program
All cows were fed concentrate diet to meet the nutritional requirement, about 2.5-3.0 % DM of liveweight and crude protein 10-12 % (Indonesian National Standard, 2009). The proximate analysis results were in Table 1.
Parameters
The parameters observed were ovarium condition (size and shape), and the development of follicles as well as the concentration of FSH and LH.
Data analysis
The data were analyzed using one-way ANOVA in the randomized complete design. The data were analyzed using descriptive analysis in the first stage and in the second stage, the data were tested for its normality using Shapiro-Wilk, if the abnormalities were detected then the data would be transformed using arcsin square root before the analysis. If there was a difference between treatments, Duncan’s multiple range tests were applied. The analyses were facilitated using SPSS software version 23.
RESULTS AND DISCUSSION
The concentration of FSH and LH
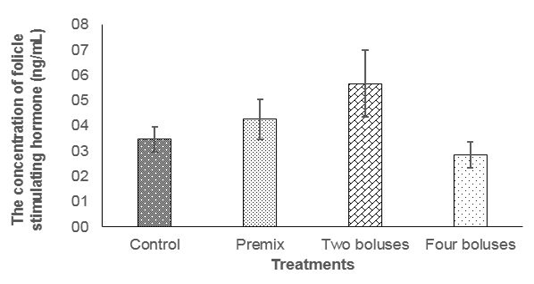
The concentration of FSH was not different between treatments (Table 2) due to probably a high variation caused by different individual responses, although the concentration of FSH tended to be higher in C group compared to A, B and D groups (Figure 1); thus, feeding bolus contain
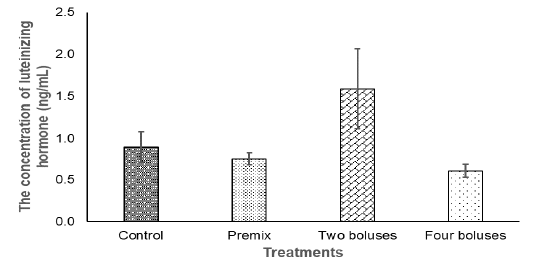
Figure 2: The concentration of the luteinizing hormone in the crossbred cows receiving different treatments.
ing herbal (grounded Moringa oleifera), vitamin A, D, E, and Zinc picolinate was enough to maintain the concentration of FSH to respond to follicle development and abnormality of estrus cycle in the reproductive tract of cows
Table 3: The concentration of the luteinizing hormone in cows offered boluses for ovarian hypofunction treatment.
| Treatments | T1 | T2 | T3 |
ng/ml | |||
| A | 0.86 ± 0.54 | 1.07 ± 1.17 | 0.76 ± 0.64 |
| B | 0.89 ± 0.30 | 0.76 ± 0.26 | 0.60 ± 0.42 |
| C | 2.17 ± 1.81 | 1.45 ± 1.53 | 1.14 ± 1.68 |
| D | 0.86 ± 0.41 | 0.46 ± 0.058 | 0.49 ± 0.224 |
Table 4: The development of follicles, the presence of corpus luteum, and the recovery level of ovarian hypofunction of crossbred cows administered with bolus supplementation.
| Treatments | T1 | T2 | T3 | Normal (Recovered) (heads) | |||
| The presence of follicles and corpus luteum (heads) | |||||||
| Right | Left | Right | Left | Right | Left | ||
| A | 0 | 0 | 2 | 0 | 3 | 4 | 4 (67%) |
| B | 0 | 0 | 6 | 5 | 5 | 3 | 5 (83%) |
| C | 0 | 0 | 6 | 4 | 6 | 4 | 6 (100%) |
| D | 0 | 0 | 3 | 3 | 3 | 6 | 6 (100%) |
Table 5: The average of follicle size (diameter) of crossbred cows administered with bolus supplementation.
| Perlakuan | T1 | T2 | T3 | |||
Follicle size (cm) | ||||||
| Right | Left | Right | Left | Right | Left | |
| A | 0 | 0 | 1.48±0.36 | 0±0.00 | 0.95±0.09 | 0.92±0.64 |
| B | 0 | 0 | 0.72±0.18 | 0.81±0.24 | 0.63±0.44 | 0.30±0.26 |
| C | 0 | 0 | 0.75±0.20 | 0.76±0.09 | 0.49±0.30 | 0.24±0.27 |
| D | 0 | 0 | 0.86±0.20 | 0.91±0.38 | 0.37±0.47 | 0.59±0.15 |
The normal concentration of daily FSH in Bos taurus cows was 4-6 ng/ml (Alvarez et al., 1998). Folicle Stimulating Hormone concentration of cows in the current experiment were 6.92± 6.17 ng/ml (T2) and 3.32± 3.61 ng/ml T3); that was higher than FSH concentration in cows at 8 hours (0.63 ± 0.08 ng/mL) before ovulation and 56 hours (0.34 ± 0.14 ng/mL) after ovulation (Tortorella et al., 2017). At the end of the current experiment (day 42), the concentration of FSH declined in every treatment, this was probably cows entered the luteal phase.
The concentration of LH in the current experiment did not show significantly different between treatment (Table 3) due to a high variation in the data that was probably caused by different individual responses, although the concentration of LH tended to be higher in the C group in comparison with A, B, and D groups (Figure 2). The concentration of LH was lower compared to basal LH, about 1.17-1.58 ng/ml (Abreu et al., 2018), when the increase of LH concentration was higher than the basal level, the cows then experienced ovulation (Figure 2). Thus, bolus containing herbal (grounded Moringa (Rahman) and vitamin A, D, E as well as Zinc picolinate supplementation were enough to maintain the concentration of LH in response to ovulation and the growth of corpus luteum, indicating a normal reproduction cycle (Thatcher, 2017). The synergies of LH and FSH stimulated the growth of follicles and normal ovulation (Raju et al., 2013). Therefore, ovarian hypofunction in cows offered bolus, recovered.
The development of follicles and the presence of corpus luteum
The development of follicles of crossbred Limousin and or Simmental cows suffering from HPO after treatment in smallholder farmers in Sumbersuko, Sumbersuko, Lumajang district were presented in Table 4.
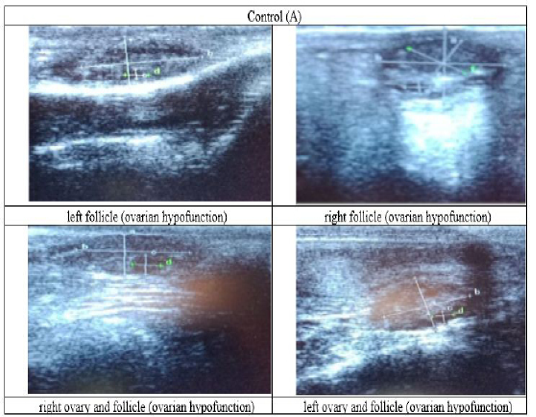
Figure 3: Ultrasound observation of the reproductive tract of crossbred cows in the control group (A).
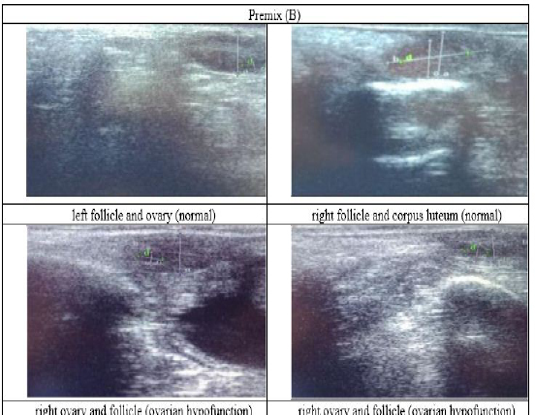
Figure 4: Ultrasound observation of the reproductive tract of crossbred cows in the premix group (B).
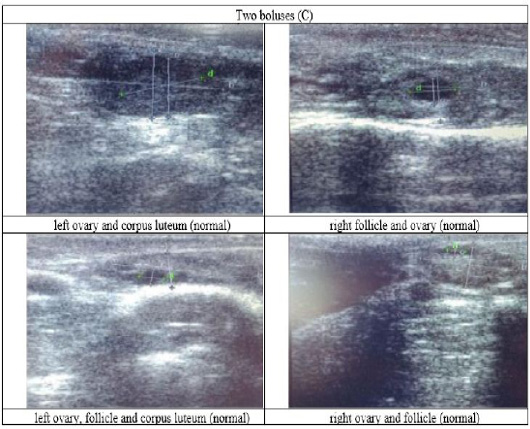
Figure 5: Ultrasound observation of the reproductive tract of crossbred cows received two boluses (C).
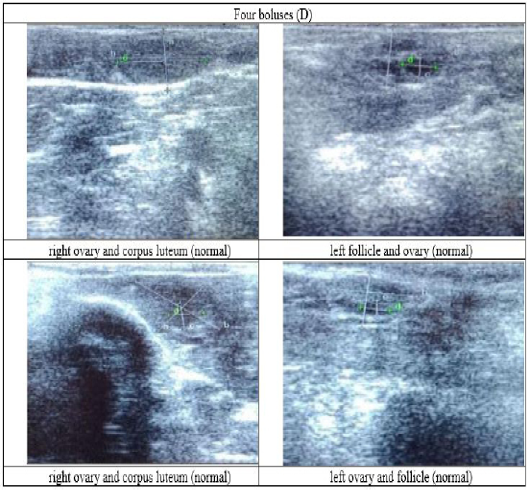
Figure 6: Ultrasound observation of the reproductive tract of crossbred cows received four boluses (D).
The cows in the control group showed the lowest follicle number, accounting for 67 % in comparison with those that were administered with premix, about 83 % (Table 4 dan Figure 3), while the follicles were presences in cows received boluses and all of them recovered from HPO (Table 4, Figure 4 and 5, while in Figure 3, the fol licles were not detected using rectal palpation and ultrasound because most of them still experienced HPO, in particular A group in comparison with B, C, and D groups. This was because they were only offered concentrate diet at 4 kg/head/day containing 77.17 % DM and 9.57 % of CP, probably was not enough (Table 1), except those that received bolus such as in C and D groups (Table 4), such a treatment using vitamin A, D, and E injection, mineral Premix (in the diet) in combination with anthelmintic could heal ovarian hypofunction within four weeks, 72.92% of them recovered, based on morphological and functional condition and follicle growth (Widarini et al., 2017). The results of the current experiment were better than results from Widarini et al. (2017) in the same breeds in terms of recovery percentage. The results of the current experiment were also better compared to a study conducted by Oktarianti (2017) who reported that after treatment the 67.7% cows were recovered, with a recovery rate was 36.5% and cows underwent AI after treatment was 38.4%, and 25.1% cows were pregnant and using hormonal treatments e.g. PGF2 Alpha or GnRH injections that showed 79 % of estrus symptoms (El-Khadrawy et al., 2015). Sarker et al. (2015) reported that the administration of GnRH, vitamin A, D, and E injection and mineral supplementation in heifers were able to normalize the ovarian cycles, which were 60.00; 46.67 and 33.33 % respectively. The recovery of the reproductive tract of cows was indicated by the development of morphological and functional ovary (the development of follicles and the presence of corpus luteum). The development of the diameter of the follicles in all treatments was presented in Table 5. The Indonesian Livestock Research Center has analyzed Moringa oleifera and showed that it had an antioxidant capacity that was able to suppress the oxidation at about 26.19 eq. vitamin C/g of leaves. The antioxidant is important for preventing free radicals in the body of animals (Chavez, 2017). The biological antioxidant was found in vitamin E that could prevent the animals from free radicals in the body’s immune system (NRC, 2000). One of strategies that could treat infertility in cows was to control the oxidative stress (OS) (Wang et al., 2013). Antioxidants could maintain the balance between the production and reactive oxygen species cleaning.. The imbalance between those factors could cause pathological concentrations in oocyte maturation, ovulation, fertilization, implantation, and embryonic development.
The observation of the follicle diameters in four different treatments did not show significantly different between treatments (Table 5), although all cows (except in A group) that showed follicle development in the observation using rectal palpation on day 21, the left follicle in A group and the corpus luteum were not detected. In Figure 3, cows in B, C, and D groups, some of them were in the luteal phase. Figures 4, 5, and 6 the corpus luteum appeared after ovulation that showed normal reproductive cycle and the luteal phase was 16-17 days followed by follicular phase 3-6 days (de Abreu et al. 2017). Therefore, supplementation with bolus healed HPO and normalize the reproductive cycle of the cows.
CONCLUSION
The best treatment for ovarian hypofunction in crossbred cows was in the using two boluses that were supplemented twice a week. It resulted in normal FSH and LH concentration and 100 % of the cows recovered.
CONFLICT OF INTEREST
The authors declare that there have no conflict of interest.
Acknowledgments
The authors would thanks to all technicians for their contribution in data collection during the experimental period and Institute of Tropical Disease (ITD) laboratory staff, in Veterinary Science Faculty, Airlangga University for the hormonal analyses. Research funds come from the Goverment Budget of the Agricultural Research and Development Agency, the Ministry of Agriculture of the Republic of Indonesia for the 2019 fiscal year with the Agricultural Research Operational Plan number 1806.208.001.051.B.1.2019.
authors contribution
Lukman Affandhy, Muchamad Luthfi and Frediansyah Firdaus shared in the study design, collection of samples, development of methodology and manuscript revision. Dian Ratnawati and Risa Antari shared in laboratory work, interpretation of data and writing the manuscript.
REFERENCES