Advances in Animal and Veterinary Sciences
Research Article
Gold Nanoparticles Based Assay for Rapid Detection of Caseous Lymphadenitis in Sheep
Marwah M Mohamed1* , Wafaa R Abdelaziz1, Rafik H Sayed2, Shasha FA2 , Abdel Fattah Ali3
1Veterinary Serum and Vaccine Research Institute (VSVRI), Agricultural Research Center (ARC), Giza, Egypt; 2Central Laboratory for Evaluation of Veterinary Biologics, Agricultural Research Center (ARC), Giza, Egypt; 3Department of Clinical Pathology, Faculty of Veterinary Medicine, Benha University, Egypt.
Abstract | Corynebacterium pseudotuberculosis is responsible for Caseous Lymphadenitis (CLA) disease in small ruminants (goats and sheep). The disease is difficult to control because antibiotic therapy is not effective. The disease is characterized by caseous abscess formation in the internal and external lymph nodes. Diagnosis is currently achieved only by routine bacteriological culture of pus obtained from external abscess. The lateral flow immunochromatographic test (LFIT) was prepared and evaluated for discover the present of Corynebacterium pseudotuberculosis in pus samples obtained from abscess in superficial lymph nodes. The minimal count of bacterial that gave positive LFIT was 102 CFU/ 0.1ml. About 100 pus samples from external lymph nodes were examined by the LFIT and the results were compared with conventional microbiological method. The obtained results demonstrate that the specificity was calculated and found to be 88.24%, while sensitivity was 90.36% and finally the accuracy was 90% of LFIT as compared to conventional microbiological method. These findings indicate that the developed LFIT test is a fast, simple and inexpensive field test of good specificity, sensitivity and accuracy that can be used to enhance Caseous Lymphadenitis control among sheep and goats.
Keywords | C. pseudotuberculosis, diagnosis, gold nanoparticle, Lateral Flow Test, sheep.
Received | February 11, 2021; Accepted | February 25, 2021; Published | March 30, 2021
*Correspondence | Marwah M Mohamed, Veterinary Serum and Vaccine Research Institute (VSVRI), Agricultural Research Center (ARC), Giza, Egypt; Email: [email protected]
Citation | Mohamed MM, Abdelaziz WR, Sayed RH, FA Shasha, Ali AF (2021). Gold nanoparticles based assay for rapid detection of caseous lymphadenitis in sheep. Adv. Anim. Vet. Sci. 9(5): 709-714.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.5.709.714
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mohamed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Caseous Lymphadenitis (CLA) is a contagious chronic disease that affects goats and sheep, caused by C. pseudotuberculosis, which is Gram-positive, non-capsulated, non-motile, non-spore forming, pleomorphic, intracellular and facultative anaerobic microorganism. CLA results in severe economic losses in goats and sheep industries worldwide especially due to reduction in milk, meat and wool production and also decreasing the fertility of the affected sheep and goats (Pekelder, 2000; Baird and Fontaine, 2007; Robles 2007).
The encapsulated abscesses are considered the most characteristic symptom of the disease. The abscesses are formed mainly in external lymph nodes but also found in visceral organs. The abscesses contain yellowish green granular thick pus (Nassar et al., 2015).The microorganism has zoonotic importance as the infected animals can contaminate meat and milk causing disease in humans characterized by development of a suppurative lymphadenitis with a chronic or recurrent course of disease (Bregenzer et al., 1997; Peel et al., 1997).The C. pseudotuberculosis bacteria can survive in the environment for long time facilitates its transmission which mainly occurs through wound contamination during sheaving season. The disease is difficult to be treated due to inability of antibiotics to reach the bacteria inside the abscesses. So, massive vaccination and accurate methods for diagnosis may be necessary to reduce the diseases prevalence (Costa et al., 2011).
The diagnosis of CLA depends upon presence of the external abscesses then confirmed by isolation and biochemical identification of the causative microorganism from pus discharge to differentiate it from the other opportunistic pathogens that also caused abscesses, such as Pasteurella multocida and Arcanobacterium pyogens (Dercksen et al., 2000; Dorella et al., 2006).The microbiological culture and biochemical testing are still the standard method for CLA diagnosis in goats and sheep. Several serological tests have been used for the diagnosis of CLA including Complement Fixation, Synergistic Inhibition of Hemolysis, Hemagglutination, Immunodiffusion and PCR (Shigidi, 1978; Burrel, 1980; Pacheco et al., 2007).Recently, the lateral flow gold nanoparticle assay is used for fast diagnosis of many important zoonotic bacterial disease such as Anthrax, tuberculosis, brucellosis etc. It has many advantages when compared with conventional and molecular detection methods, such as the low coast, ease to use in field, the results can be detected in short time (5- 10 min) (Arun et al., 2014; Kong et al., 2017).
So, the current work was conducted to prepare a gold nanoparticle lateral flow based assay for rapid diagnosis of Caseous lymphadenitis in sheep and comparing it with conventional method for bacterial isolation.
MATERIAL AND METHODS
Bacterial strain
The C. pseudotuberculosis strain was isolated from native breed of sheep suffered from external abscesses and identified bacteriologically and biochemically according to Quinn et al. (1994) and Koneman et al. (1997) and it was used for bacterin preparation.
Preparation of whole C. pseudotuberculosis inactivated cells
The preparation of formalin inactivated C. pseudotuberculosis bacterin was done according to Brown et al. (1986). The bacterial cells were cultivated on selective media (5% sheep blood agar) and then single colony was inoculated in 100 ml of Brain heart infusion (BHI) broth (DIFCO, Detroit, Mich., USA). Finally, the obtained broth was transferred to 500 ml of BHI broth and incubated at 37⁰C for 24 hours in shaking incubator. The cells were inactivated with formalin (0.2%, vol/vol) and incubated for 24 hrs at room temperature. The bacterial cells were collected by centrifugation (3000 rpm for 15 min) and then washed with Phosphate Buffer Saline (PBS) twice. Bacterial concentrations were determined by McFarland’s standards tubes, adjusted to1010 bacteria/ml by using sterile PBS. 50µl of bacterin was cultivated on 5% sheep blood medium to ensure the sterility of bacterin.
Preparation of Polyclonal antibodies
The polyclonal antibodies against whole inactivated C. pseudotuberculosis cells were prepared according to Cameron and Maria (1971). One ml of prepared whole cell bacterin was added to one ml of Complete Freund’s Adjuvant (CFA, Sigma, UK) and mixed well, then injected subcutaneously into rabbit. The injections were given on days 1,7and 14.The serum was collected on day 28 post-injection and stored in -20°C until used.
IgG purification from the polyclonal antibodies
The purification was performed using the Caprylic acid (Elke et al., 2008). Rabbit serum was centrifuged at 10000 xg for 30 min. Supernatant was collected and pH was adjusted to 4.6 with acetic acid 1.76 N. Caprylic acid was added drop wise to a final concentration of 2.5% with vigorous stirring for one hour followed by centrifugation at 10000 xg for 30 min. The obtained supernatant was dialyzed against PBS buffer at 4˚C with several buffer changes.
Synthesis of colloidal gold nanoparticles solution
The preparation of 40 nm gold nanoparticles colloidal solution was performed by the citrate reduction method according to Li et al. (2011).The diameters of the performed gold nanoparticles were tested by using spectrophotometer scanning at 400-600nm.
Conjugation of the purified rabbit IgG with gold nanoparticles
The conjugation of rabbit IgG with gold nanoparticles was done according to Kong et al. (2017).
Development of Lateral Flow Immunochromatographic Test (LFIT) strips
The LFIT strips were prepared according to Shaimaa et al. (2018) and included the preparation of Sample pad, Conjugation pad and Nitrocellulose (NC) membrane.
Determination of specificity, sensitivity and validity of the performed LFIT
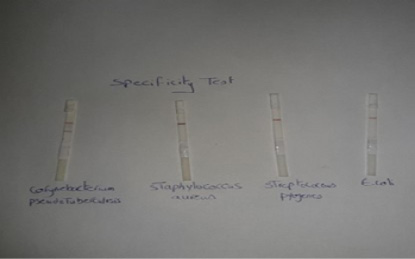
Specificity of the performed LFIT: Standard strains form other pyogenic bacteria that cause abscess formation such as Staphylococcus aureus, Escherichia coli and Streptococcus pyogens were examined by using LFIT stripes.
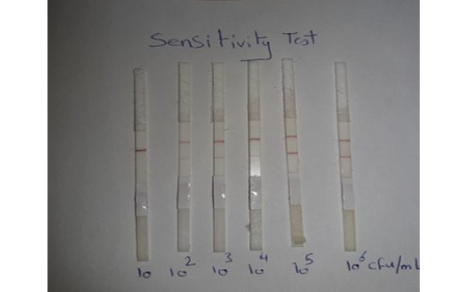
Sensitivity of LFIT: The local C. pseudotuberculosis ovis strain was tested and diluted (10-1, 10-2, 10-3, 10-4, 10-5and 10-6) and the minimal concentration of bacterial cells that gave positive in prepared LFIT was determined.
Validity test of LFIT for detection of C. pseudotuberculosis compared with bacteriological examination: A total of 100 pus samples were obtained from sheep (from Qualiobia governorate) suffering from abscessed superficial lymph nodes. The obtained samples were subjected to examine by LFIT stripes in comparison with bacteriological isolation as described in Table (1).
Table 1: Evaluation of LFIT by using direct bacteriological examination
|
Bacteriological examination |
||||
|
LFIT
|
Positive (Diseased) |
Negative (not diseased) |
Total | |
| + |
A (T+) |
B (F+) |
a + b | |
| - |
C (F-) |
D (T-) |
c + d | |
| Total | a + c | b + d |
a + b + c + d (n) |
|
(F+): False positive; (F-): False negative; (T+): True Positive; (T-): True negative.
Statistical analysis: Mean, standard error of mean and standard deviation of LFIT and bacteriological examination results were determined according to Thrusfield (2007).
Determination of specificity, sensitivity and accuracy of LFIT (Thrusfield, 2007)
Specificity: Is the ability of Lateral flow diagnostic kits to correctly identify the percentage of the samples not containing C. pseudotuberculosis:
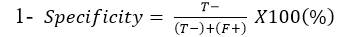
Sensitivity: Is the ability of Lateral flow diagnostic kits to correctly identify the percentage of the samples containing C. pseudotuberculosis:
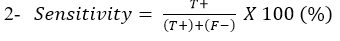
Accuracy: Describe the degree to which measurement reflects the true status of what is being measured.
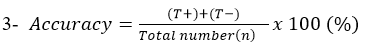
RESULTS AND DISCUSSION
Caseous lymphadenitis or cheesy gland is a chronic suppurative disease caused by C. pseudotuberculosis that is a member of Mycobacterium, Corynebacterium, and Nocardia group which includes the species of medical, biotechnological and veterinary importance. C. pseudotuberculosis mainly affects small ruminants (sheep and goats) but it also affects equine, bovine, camels and humans, showing zoonotic important (Bastos et al., 2012). The CLA causes great economic losses in sheep and goats.
C. pseudotuberculosis identification is usually carried out using conventional methods such as microscopy, cultivation on selective media (sheep blood agar), biochemical or serological tests or molecular techniques such as PCR, gene sequencing etc. (Oh et al., 2005; Kaittanis et al., 2009). The conventional methods are time consuming, whereas modern molecular techniques are expensive and unsuitable for field condition. Many scientists are interested in the lateral flow assay due to their high sensitivity, high specificity, cheap and easy application by non-specialized personnel and highly stable for a prolonged time without need for refrigeration. The lateral flow assay depends on an immunochromatographic procedure that utilizes antigen– antibody properties (Moongkarndi et al., 2011; Heidy et al., 2020). Therefore, the current work was aimed to prepare LFIT strips and also to evaluate its specificity, sensitivity and accuracy for rapid detection of C. pseudotuberculosis and comparing it with conventional bacteriological isolation.
Sensitivity results for performed LFIT was shown in Figure (1), the minimal concentrations of C. pseudotuberculosis that could generate positive bands on the test strip was 102 CFU/ 0.1 ml. while, the results obtained from Chirathaworn et al. (2011) showed that the sensitivity of leptospira was 10 CFU /ml by using lateral flow method. Moreover, Moongkarndi et al. (2011) found that the test strips of lateral flow can detect S. Enteritidis and S. typhimurium in a culture medium at levels as low as 106 and 104 CFU /ml, respectively. Finally, Wiriyachaiporn et al. (2013) found that the sensitivity of lateral flow method for S. aureus was 106 CFU/0.1ml.
The results in Figure (2) demonstrated that the Lateral flow test gave positive (red line) for C. pseudotuberculosis and gave negative for the other pyogenic bacteria that cause abscess formation including Staphylococcus aureus, Streptococcus pyogens and Escherichia coli. The obtained results were indicated that the prepared LFIT
Table 2: Validation test for LFIT for detection of C. pseudotuberculosis as comparing with bacteriological examination.
| TEST | Bacteriological examination | Specificity test (%) | Sensitivity test (%) |
Accuracy test (%) |
||
|
+ve |
-ve |
Total |
||||
| LFIT |
88.24 |
90.36 |
90 |
|||
| +ve | (T+)75 | (F+)2 | 77 | |||
| -ve | (F-)8 | (T-)15 |
23 |
|||
| Total | 83 | 17 |
100 |
|||
The results in Figure (2) demonstrated that the Lateral flow test gave positive (red line) for C. pseudotuberculosis and gave negative for the other pyogenic bacteria that cause abscess formation including Staphylococcus aureus, Streptococcus pyogens and Escherichia coli. The obtained results were indicated that the prepared LFIT has high specificity in its reaction and showed no cross reactivity with the other bacteria that cause abscess formation. These findings disagreed with Shaimaa et al. (2018) who showed that the performed LFIT has low specificity and was unable to differentiate between Salmonella Enteritidis and Salmonella pullorum as there is cross-reactivity between them as they share the same somatic antigen.
Abscessed sheep lymph node samples were examined by the developed LFIT and bacteriological isolation. The obtained results were analyzed and compared to detect the accuracy, specificity and sensitivity. As cleared from Table (2), that (T+) 75, (F+) 2, (F−) 8 and (T−) 15. Also, the results of specificity was 88.24%, while sensitivity was 90.36% and finally the accuracy was 90% when compared with bacteriological isolation for detection of C. pseudotuberculosis. These results were near to that obtained by Shaimaa et al. (2018) who found that the specificity, sensitivity and accuracy of LFIT in detection of Salmonella Enteritidis were found 80%, 91% and 90% respectively. While, Heidy et al. (2020) found that the specificity and sensitivity of LFIT kit compared to PCR in identification of Mycoplasma gallisepticum was found to be 91.18% and 56.6%, respectively. Moreover, Kato et al. (2004) recorded that the LFIT specificity was 95.8%, while accuracy rate was 94.0% and finally the sensitivity was 90.6% for detection of Helicobacter pylori in children. Finally, Soliman et al. (2020) showed that the specificity, sensitivity, and accuracy of the LFIT for detection of C. perfringens toxins were 95.2%, 81%, and 90%, respectively for α toxin. While, they were 98.5%, 76.6%, and 72%, respectively for β toxin and finally they were 98.8%, 66.6%, and 95%, respectively for ε toxin.
CONCLUSION
The developed gold nanoparticles lateral flow method for detection of C. pseudotuberculosis was found not only suitable as a screening field test but also very rapid; requires only 5 minutes, simple, cheap, does not require special equipments or electricity and can discover even the dead bacterial cells in pus samples, as the isolation of C. pseudotuberculosis is not easy. The test can be performed by modestly trained person and gives results that can help in detection of Corynebacterium pseudotuberculosis and diagnosis of Caseous lymphadenitis.
ACKNOWLEDGEMeNTS
The authors would like to thank the Veterinary Serum and Vaccine Research Institute (VSVRI), Cairo, Egypt for supporting this research.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
AUTHOR’S CONTRIBUTION
All authors contributed equally.
REFERENCES