Advances in Animal and Veterinary Sciences
Research Article
Anatomical and Histological Features of the Gastrointestinal Tract in the Nile Crocodile, (Crocodylus Niloticus) with Special reference to its Arterial Blood Supply
Nora A. Shaker, Asmaa M. Ibrahium*
Anatomy & Embryology, Faculty of Veterinary Medicine, Cairo University, Egypt.
Abstract | The present study was investigated the anatomical features of the gastrointestinal tract in the Nile crocodile with detailed information about its arterial blood supply. Four adult Nile crocodiles were used for studying the morphology, histology of digestive tract and its arterial supply. The crocodile was euthanized then fixed in situ with 10% formalin to be dissected. Other fresh specimen was sectioned into small pieces for histological investigation. Two specimens were injected by colored latex neoprene through the heart for arterial study. The gastrointestinal tract composed of long straight esophagus, two sacs stomach, and double looped duodenum occupying pancreas, coiled small intestine and colorectum. The arterial blood supply originated from the celiacomesentric artery which gave up three branches; right gastric, intestinal and gastro duodenal arteries. The mucosal lining of the all digestive tract was simple columnar epithelium varied from secretory to absorptive, lamina propria was mostly fibroelastic connective tissue contains either glands or lymphocytes. Tunica musculosa frequently formed of inner circular and outer longitudinal smooth muscles excluding the fundic part of stomach had middle layer.
Keywords | Anatomy, Arterial supply, Gastro-intestinal tract, Histology, Nile crocodile.
Received | October 14, 2020; Accepted | December 12, 2020; Published | March 30, 2021
*Correspondence | Asmaa M Ibrahium, Anatomy & Embryology, Faculty of Veterinary Medicine, Cairo University, Egypt; Email: [email protected]
Citation | Shaker NA, Ibrahium AM (2021). Anatomical and histological features of the gastrointestinal tract in the nile crocodile, (crocodylus niloticus) with special reference to its arterial blood supply. Adv. Anim. Vet. Sci. 9(5): 692-699.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.5.692.699
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Shaker and Ibrahium. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The digestive system of vertebrates presents structural and functional adaptations to their various alimentary routine, in addition to the distinct conformational aspects between herbivores and carnivores (Secor, 2005). The Nile crocodile considered as one species of reptiles having a greater detectible variation in their gastrointestinal tract, making veterinary diagnostics difficult (Mitchell and Diaz-Figueroa, 2005). Reptiles considered as useful simulations for studding the regulation of gastrointestinal tract; they have extreme regulatory responses to feeding so they are better than experimental mammalian models (Secor and Diamond, 1998).The alimentary tract of crocodile reptiles is similar to that of higher vertebrates except for some structures showing adaptive modifications according to the nature of their feeding.
There are little attention in the literature about descriptive anatomy of digestive system of the Nile crocodile and its arterial blood supply, the objective of this study was presenting the anatomical and histological structures of gastrointestinal tract, including the distribution of its arteries with a defined nomenclature to improve the present information and to compare our findings with the other reptiles. Also this data was useful for learning the functional physiology of the digestive process. This knowledge would be necessary for veterinary medical diagnosis and medical experimentation (Vander Merwe and Kotze, 1993).
Material and methods
The experimental protocol was approved by the ethical committee of the faculty of Veterinary Medicine Cairo University. (The approval No: Vetcu16072020203).
Animals
The present study was conducted on four adult Nile crocodiles. The Nile crocodile (Crocodylus niloticus) belong to family crocodylidae, native to freshwater habitats in Africa; it was obtained from Aswan, Nasser Lake. It was euthanized by using sodium pentobarbital 60-120 mg/kg intraperitoneal cause death (Perry, 1990).
Anatomical examination
One specimen for morphological study, where it was fixed in situ with 10% formalin then dissected to reveal the different parts either external or internal features of gastrointestinal tract. Two specimens were injected by colored latex neoprene through the heart for arterial study. The specimens were left in a mixture of 10% formalin 4% phenol and 1% glycerin for two days before routine dissection. The obtained results were photographed. The nomenclature used in this study was that given by (Nomina Anatomica Veterinaria, 2017).
Histological examination
Other fresh specimen was sectioned into small pieces for histological investigation, theses sections were fixed in Bouin’s fluid and others soaked in neutral buffer formalin 10%. The blocks dehydrated in ethanol, cleared by xylene and embedded in paraplast. Consecutive segments were cut at a thickness 5-6 μm using rotatory-microtome and stained with H&E (Bancroft and Gamble, 2008).
Results
Morphological study
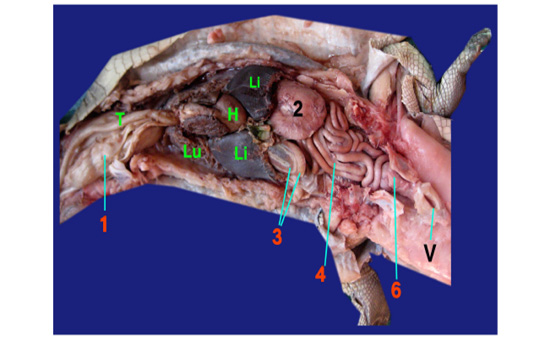
The Esophagus: It was a straight muscular tube about 7-8cm in length, began from the short pharynx connected with aditus opesophagus, coursed dorsally to the trachea, passing between the tracheal bifurcation, the right and left lungs and two lobes of liver, then terminated vertically in the right side of stomach at level of 8-9th ribs (fig. 1, 2, 3/1). The mucous membrane of esophagus had straight 30-40 muscular folds (fig. 2/1a).
The Stomach: (fig. 1/2) it was formed by two portions; a large sac represented as c- shape which had a greater curvature facing to the left side while the lesser curvature formed a small angle between the cardia and pylorus (fig. 2,3/2a). Small sac was spherical in shape located in the lesser curvature at the right side (fig. 2,3/2b). Two parts of stomach was connected by a strong sphincter (fig. 2/S). It had two opening; the cardiac opening (fig. 2/2c) presented in the cranial end of the stomach and pyloric opening (fig. 2/2d) located between the small sac of the stomach and inner loop of the duodenum. The internal structure of large sac of stomach was formed by thick convoluted folds (fig. 2/2e) while smooth mucosa in the small sac (fig. 2/2f).
The small intestine: it was divided into three segments: the duodenum, jejunum and ileum which had no a macroscopic demarcation between them (fig. 1, 2, 3/3), (fig 1, 2, 3,/4) and (fig. 3/5) respectively. The duodenum was located on the right side, forming a double u-shaped loop, about 12-13cm in length (fig. 1, 2, 3 /3). The first inner loop of the duodenum (fig. 2, 3/3a) extended from the pyloric region of stomach to the second outer loop of the duodenum (fig. 2, 3/3b). The duodenum was occupying the pancreas in its dorsal surface. The jejunum was formed by numerous coils about 35- 45cm in length that occupied the right and left side of the coelomic cavity and attached to the dorsal body wall by long mesentery (fig. 1, 2, 3/4) ,it began from the outer loop of duodenum and terminated in the ileum with slightly narrowing in diameter. The duodenum appeared externally thicker than the jejunum and the ileum (fig. 3/5).
Legend of figures (1 to 3):
1)Esophagus, 1a)Mucus membrane of esophagus, 2)Stomach, 2a)Large sac, 2b)Small sac, 2c)Cardiac opening, 2d)Pyloric opening, 2e)Convoluted folds of large sac, 2f)Smooth mucosa of small sac, 3)Duodenum, 3a)Inner loop, 3b)Outer loop, 4)Jejunum, 5)Ileum, 6)Colorectum, 6a)Cranial chamber, 6b)Caudal chamber, 6c)Straight part of colorectum, 6d)Raised fold, 6e)Transverse fold, Sp)Strong sphincter, C)Internal crypt, P)phallus, H)Heart, Li)Liver, Lu)Lung, T)Trachea, V)Vent.
The large intestine represented as colorectum that appeared by sudden change in diameter of the narrow ileum (fig. 1, 3/6). The colorectum composed of two parts; started with s-shaped and terminated by a straight part extended to cloaca (fig. 3/6c). The s shaped of colorectum divided into two chambers with external constriction in between. The cranial chamber (fig. 3/6a) formed internally by crypt (fig. 3 /C) separated from caudal part (fig. 3/6b) by strong sphincter (fig. 3/ Sp), more over the caudal one lined firstly by 15-20 raised folded (fig. 3/6d) followed by smooth area. The s shaped and straight part of colorecum (fig. 3/6c) demarcated internally by transverse fold (fig. 3/6e). The straight part of colorectum included the phallus (fig. 3/P) internally and opening of ureter just caudally to transverse fold and it mucosa carried a small straight folded.
Histological investigation (fig. 4):
The mucosal lining of the esophagus formed of simple columnar cells, its lamina propria was fibro elastic connective tissue with well-developed lamina muscularis mucosa. Tunica sub mucosa composed of loose C.T. and tunica musculosa arranged as inner circular and outer longitudinal smooth muscles (Fig. 4).
Stomach was divided into two parts; fundic (large sac) and pyloric part (small sac). The fundic and pyloric surface was lined by simple columnar secreting epithelium. The propria sub mucosa of fundic region was fibroelastic c.t. contained abundant long, straight and branched gastric gland while it was simple and coiled in pyloric part. Tunica musculosa of fundic was thick formed from three layers outer, inner circular and middle longitudinal muscles, but in pyloric region it was inner circular and outer longitudinal muscle fibers (Fig. 4).
The duodenal mucosa carried intestinal villi lined by absorptive simple columnar epithelium. The lamina propria sub mucosa was fibro elastic connective tissue rich in duodenal glands. Tunica musculosa consisted of thick inner circular and outer longitudinal smooth muscle fibers (Fig. 4).
The epithelial lining of jejunum and ileum was absorptive simple columnar with goblet cells; except the intestinal villi in ileum was lower than jejunum. The lamina propria contained fibro elastic connective tissue with heavy infiltrations of lymphocytes. Tunica musculosa formed of inner circular and outer longitudinal muscles (Fig. 4).
The colon mucosa was highly folded and branched lined by simple columnar epithelium with numerous goblet cells. Lamina propria had abundant mucous gland. Tunica submucosa formed of loose connective tissue with nerve plexus. Tunica musculosa was thick coat of circular smooth muscles (Fig. 4).
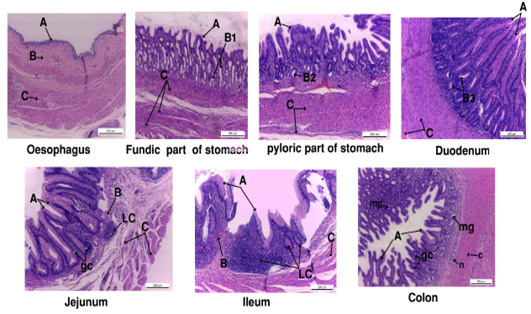
Figure 4: Photograph showing the histology of gastro-interstinal tract. A, tunica mucosa ; B, tunica submucosa; B1, fundic gland; B2, pyloric gland; B3, duodenal gland; gc, goblet cell ; LC, lymphocyte; mg, mucous gland.
Arterial blood supply
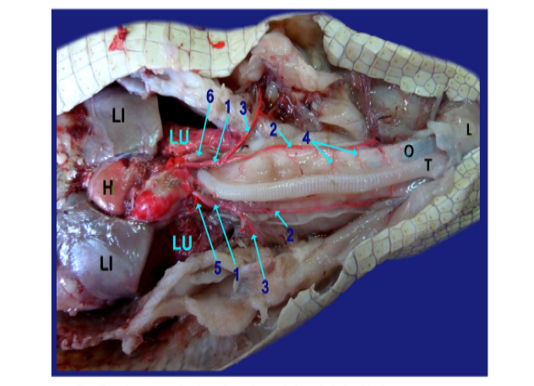
The left ventricles gave at the heart base; the aorta and the brachiocephalic artery (fig.5 /1). The latter one divided into the common carotid (fig.5/2) and the subclavian artery (fig.5/3). The common carotid artery gave several esophageal branches to esophagus (fig.6/4).
The left and right aorta (fig. 5, 6, 8/5, 6 respectively) formed the aortic arches at the level of the tracheal bifurcation then ran caudally to unite by a short communicating branch just cranial to the right side of the stomach (fig. 6,8/7) .
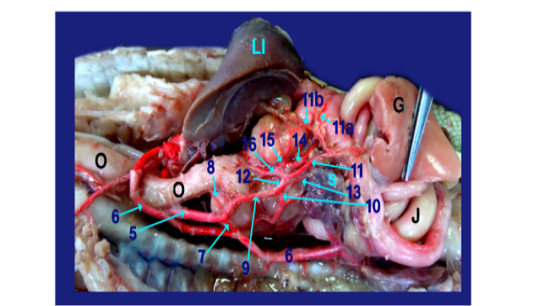
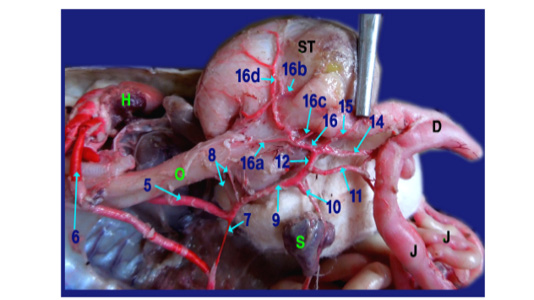
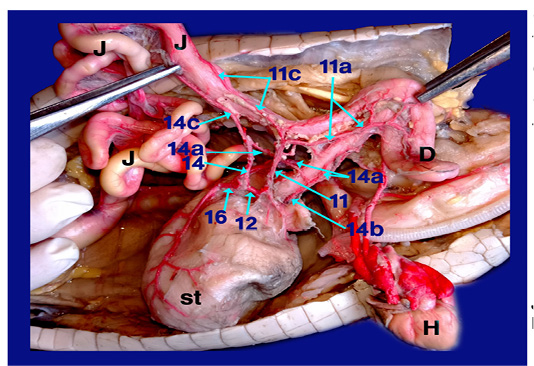
The right aorta continued as the dorsal aorta while the left aorta gave 5-6 branchesto the terminal part of esophagus (fig.6, 8/8) then, continued as the celiacomesentric artery (fig.5, 6, 7, 8/9) which gave up three branches; the right gastric (fig.6,7, 8/10), the intestinal (fig.6, 7, 8, 9/11) and the gastro duodenal arteries (fig.6, 8, 9 /12). The last two arteries arose by common trunk.
Legand of figures (5 to 9):
1)Brachicephalic A., 2)Common Carotid A., 3)Subclavian A., 4)Esophageal branches of common carotid A., 5)Left aorta, 6)Right aorta, 7)Short communicating branch, 8)Branches to the terminal part of esophagus, 9)Celiacomesentric A., 10)Right gastric A., 10a)Branch to cardiac region of stomach, 10b)Branch to body of stomach, 10c)Branch to caudal end of stomach, 11)Intestinal A., 11a) Branch to1st part of duodenum and outer loop, 11b) Hepatic branch, 11c)Dorsal jejunal branch, 12)gastroduodenal A., 13)Splenic A., 14)duodenal A., 14a) Branch supplied the 2nd loop of duodenum., 14b)Fine branches of duodenal artery to the small sac of stomach, 14c) branches to ventral wall of initial part of jejunum 15)Pyloric A., 16)left gastric A., 16a) branches of left gastric artery to cardiac region, 16b) branches of left gastric artery to small portion of stomach, 16c) branches of left gastric artery to pyloric region, 16d) Twigs of left gastric artery to the body of stomach, 17) Jejunal A., 18) Colic branches.
H) Heart, Lu)Lung, T)Trachea, L)Larynx, O)Esophagus, St)Stomach, D)Duodenum, J)Jejunum, C)Colorectum, V)Vent, S)spleen, Li)Liver, G)Gonad.
The Right gastric artery gave splenic artery (fig.6, 7/13) then split into three main branches on right face of stomach; branch to the cardiac region, one to the body and other to the terminal caudal end of stomach (fig. 7 /10a,10b and 10c respectively).
The intestinal A. supplied 1st part of duodenum (fig. 6, 9/11a) with its second loop (outer loop) included pancreas and gave hepatic branches (fig. 6/11b) to right and left lobe of liver. It ran on the dorsal wall of jejunum (1st dilated part about 4cm) as dorsal jejunal branch (fig.9/11c).
The gastro duodenal artery gets out duodenal A., Pyloric A. and left gastric A. (fig. 6, 8, 9 /14, 15 and 16 respectively).
A) Duodenal A. (the largest branch) (fig. 6,8,9 /14) supplied the 2nd loop of duodenum (inner loop) (fig.9/14a), pancreas and gave several fine branches to the small sac of stomach (fig. 9/14b). It also gave ventral jejunal branch (fig. 9 /14c) to the ventral wall of initial part of jejunum.
B) Pyloric A. (fig. 6, 8 /15) supplied the terminal part of stomach (pyloric region) then continued to the right lobe of liver.
C) Left gastric A. (fig. 6, 8, 9 /16) passed to the left face of stomach giving several fine branches (fig. 8 /16a) to cardiac region, small portion of stomach (fig. 8 /16b) and pyloric region (fig. 8/16c), also gave few twigs (fig. 8 /16d) to the body of stomach.
Right aorta (fig. 6, 7, 8/6) gave jejunal A. (fig. 7/17) which divided into 5-6 primary jejunal branches which gave secondary jejunal branches and finally terminated as intestinal arches.
Splenic artery (fig. 6, 7/13) was a branch from the right gastric artery which gave proper splenic branch in its hilus then anastomosed with last jejunal artery supplying the terminal part of jujenum (ileum before change in diameter from narrow to wide) then passed on the dorsal aspect of colon giving colic branches (fig. 7 /18) to supply the dorsal aspect of colorectum till the cloaca.
Discussion
Morphological study
The esophagus was straight muscular tube, began from the pharynx, passing between the tracheal bifurcation that our results as mentioned by Lahsik (2012) in Uromastyx aegyptiaca and Chamaeleon africanus, Romao et al. (2011) in Caiman crocodilus, Melanosuchus niger and Paleosuchus palpebrosus and Sartori (2009) for Hemidactylus mabouia. The mucous membrane of esophageus had muscular folds that agreed with the studies on Alligator mississippiensis, other crocodilians and mammals (Uriona et al., 2005; Theodoropoulos & Ledford, 2000).
The Stomach was formed by two portions; large sac in left side and small spherical one in right side, that result differing from that given by Lahsik (2012) in Uromastyx aegyptiaca and Chamaeleon africanus; Romao et al. (2011) in Caiman crocodilus, Melanosuchus niger and Paleosuchus palpebrosus, whom stated that whole stomach was single sac situated at the left side of the abdominal cavity. Also Mitchell & Diaz-Figueroa (2005) in Nile crocodile referred to the presence of pyloric dilatation. The large sac of stomach represented as C shape while in snakes and lizards (Goin & Gain, 1971) was elongated.
The duodenum located as a double U shaped loop with the pancreas in its dorsal surface as described by (Stahl, 2003; Van der Merwe and Kotze, 1993) in Nile crocodile, whereas in Alligator mississippiensis (Evans, 1986) it had only a single loop. Our study revealed that the jejunum was formed by numerous coils occupied the right and left side of the coelomic cavity and attached to the dorsal body wall by long mesentery and terminated in the ileum without external demarcation between them, these similar to Romao et al. (2011) in Caiman crocodilus, Melanosuchus niger and Paleosuchus palpebrosus, Van der Merwe and Kotze (1993) in Nile crocodile and Alligator mississippiensis (Evans 1986).
In accordance with Romao et al. (2011) and Leonardi et al. (2002), the wall of the duodenum was visibly thicker than the other parts of small intestine. Also Van der Merwe and Kotze (1993) in Nile crocodile reported that ileum and jejunum were of equal length, but it not recorded in our result. Lahsik (2012) reported that the small intestine composed of two part; duodenum and ileum.
Our study approved that the caecum was absent in the Nile crocodile and carnivores reptilian species as reported in (Abdeen et al.,1994) but herbivores reptiles as in lizards (Uromastyx aegyptiaca) and snakes Chamaeleon africanus had a caecum in right side beside the colon that mentioned by (Lahsik, 2012; Goin and Gain 1971) that correlated to mode of feeding. The large intestine appeared by sudden change in diameter of the narrow ileum, agreed with Romao et al. (2011) in Paleosuchus palpebrosus and Van der Merwe and Kotze (1993) in Nile crocodile. The large intestine represented as colorectum that result similar to (Ibrahim, 2013; Shaker, 2013) in fish but Shaker (2010) reported in dog, the large intestine composed of colon and rectum. Our result added it composed of two parts started with S shaped and terminated by a straight part extended to cloaca.
Histological investigation
The esophageal mucosa formed of simple columnar cells. That result approved by Elliott (2007) in reptiles and fish species (Parillo et al., 2004), while Ahmed et al. (2009) in Varanus niloticus and Lahsik (2012) in lizard, it was ciliated simple columnar cells. However the lining was stratified squamous epithelium in mammals (Eurell and Frappier, 2006) and was keratinized stratified squamous epithelium in fowl (Hamdi et al., 2013). In agreement with Lahsik (2012), the tunica musculosa organized as inner circular and outer longitudinal smooth muscles; in contrast with fowl (Hamdi et al., 2013) as the two layers was inner longitudinal and outer circular layer.
In line with Ahmed et al. (2009) in Varanus niloticus, the stomach was divided into fundic and pyloric part and its surface epithelium was secreting simple columnar cells. Tunica musculosa of fundic region formed from three layers outer, inner circular and middle longitudinal muscles, although it was reported by (Lahsik, 2012 in lizard and Ahmed et al., 2009 in Varanus niloticus) as two layers; inner circular and outer longitudinal muscle fibers.
As similar as Ahmed et al. (2009); Elliott (2007) in Nile crocodile the duodenal mucosa was in the form of long abundant villi covered by absorptive simple columnar epithelium, tunica musculosa consisted of thick inner circular and outer longitudinal smooth muscle fibers. The epithelial lining of ileum was absorptive simple columnar with goblet cells. Lamina propria has heavy lymphocytic infiltrations. Tunica musculosa formed of inner circular and outer longitudinal muscles, that finding agreed with Ahmed et al. (2009) and Elliott (2007).
The colon mucosa was lined by simple columnar epithelium with several goblet cells. Tunica submucosa formed of loose connective tissue with nerve bundles and blood vessels. That investigation was mentioned by Ahmed et al. (2009) in Varanus niloticus. The Tunica musculosa was thick coat of circular smooth muscle, although Ahmed et al. (2009) detected that, it was inner thick circular and outer thin longitudinal.
Arterial blood supply
The present study was investigated that Nile crocodile had heart with four chambers like bird and mammal and differ from other reptiles as lizards, turtles and snakes had three chambers; two atrium and one ventricle that agreed with Bekdemir (2013). In accordance with Farmer et al (2008) in American alligator, Kardong (2006) and Grigg (1991), our result noted that the left and right aorta formed the aortic arches then united caudally by a communicating branch just cranial to the right side of the stomach and the right aorta continued as the dorsal aorta.
In our study we documented that, esophagus was supplied by several branches from common carotid artery and left aorta that agreement with (Dyce et al., 2010) who said the oesophagus supplied by oesophageal branches from common carotid artery, thoracic aorta and abdominal aorta. Otherwise Maher and Reem (2018) reported in Guinea pig that parietal branch of left gastric artery responsible for supplying esophagus.
Regarding to our observation, the celiacomesentric artery supplied the gastrointestinal of crocodile that similar to (Santos et al., 2007) in fishes. This asimilar to Farmer 2008 reported the celiac artery was supplied the stomach, spleen, liver, pancreas and duodenum. While the dorsal aorta (right aorta) gave the mesenteric artery supplied the intestine.
According to our investigation, the left aorta continued as celiacomesentric artery which gave up the right gastric, intestinal and gastro duodenal arteries, this results differed from Santos et al. (2007) the celiacomesenteric artery of bony fishes emerged from the dorsal aorta (Axelsson et al., 1991) in estuarine crocodile (Crocodylus porosus) the celiac artery was derived from the right aorta and Farmer et al. (2008) in American alligator stated that celiac artery was the direct continuation of the left aorta which gave spleno-intestinal, gastro hepatico-intestinal and pancreo-intestinal artery.
The intestinal tract was supplied by the celiacomesentric artery, through the intestinal artery and gastro-duodenal artery this study was disagreement with Farmer et al. (2008) in American alligator mentioned that, it was supplied by the right aorta through the mesenteric artery. Our result recorded the right aorta gave jejunal arteries. Moreover splenic artery, after emerged from its hilus, anastomosed with last jejunal artery supplying the terminal part of jujenum and the dorsal aspect of colorectum as colic branches that a similar to Dyce et al. (2010) in domestic animals mentioned the jejunal arteries derived from the cranial mesenteric artery while colic artery come from caudal mesenteric artery.
Conclusion
Our study investigates that gastrointestinal tract of Nile crocodile differs from other reptiles, especially herbivores and carnivores’ ones; moreover it helps veterinarians to collect data and publish baseline materials for the anatomy of Nile crocodile.
Acknowledgments
The authors thank Dr. Yasmine H. Ahmed at Department of Histology for preparing the histological specimens and helping in reading slides.
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
This study was performed in accordance to Institutional Animal Care and Use Committee (IACUC) of Faculty of Veterinary medicine Cairo University. (The approval No: Vetcu16072020203).
authors contribution
NAS and AMI designed the study and wrote the manuscript equally. NAS reviewed the result. NAS and AMI edited and approved the final manuscript.
References