Advances in Animal and Veterinary Sciences
Research Article
The Use of Biological Antimycotoxin in Amelioration of Ochratoxicosis in Broiler Chicken and Decrease of Toxin Residues in the Breast Muscles
Doaa IA Mostafa1*, Ebtisam N El Shamy2, Eman SA Mostafa3, Neveen A Rasheed4
1Department of Clinical Pathology, Animal Health Research Institute, Zagazig branch, Agriculture Research Center (ARC), Egypt; 2Department of Immunity, Animal Health Research Institute, Dokki, Giza, Agriculture Research Center (ARC), Egypt; 3Department of Chemistry, Toxicology and Nutritional Deficiency, Animal Health Research Institute, Zagazig Branch, Agriculture Research Center (ARC), Egypt; 4Department of Microbiology (Immunity), Animal Health Research Institute, Zagazig branch, Agriculture Research Center (ARC), Egypt.
Abstract | Ochratoxin A (OTA) is one of the most important and deleterious mycotoxins. The present study explored the effects of OTA on broiler by detection of the hematological and immunological changes, as well as performing kidney function tests and determining its residues in the breast muscle with a trial using biological anti-mycotoxin to eliminate OTA residues and their harmful effects. Sixty broiler chickens were used for detecting the effects of OTA. The birds were divided into three equal groups. The first group was kept as control, the second was fed with a ration containing 0.2 mg /kg OTA, and the third one was fed with a ration containing 0.2 mg /kg OTA and biological anti-mycotoxin (Bio-Stress) (1gm/liter of drinking water) from one day old until the appearance of OTA toxicity symptoms in the second gp., which was at 32 days-old. The present study revealed anemia and leucopenia in the OTA-treated group, with a significant decrease in lymphocyte level, phagocytic activity, lysozyme concentration, total protein, serum albumin, globulin; and alph-1, alpha-2, and beta, gamma globulins and calcium levels; but serum A/G ratio, creatinine, uric acid, and inorganic phosphorus levels were significantly increased. Improvement of most of these parameters occurred by the use of biological anti-mycotoxin.We detected significantly high levels of ochratoxin residue in the breast muscle of the birds treated with OTA only as compared with the control group and that treated with OTA and anti-mycotoxin.
Keywords | Broilers, Ochratoxin A, Biological anti-mycotoxin, Phagocytosis, Muscles Residues
Received | December 05, 2020; Accepted | December 17, 2020; Published | January 15, 2021
*Correspondence | Doaa IA Mostafa, Department of Clinical Pathology, Animal Health Research Institute, Zagazig branch, Agriculture Research Center (ARC), Egypt; Email: [email protected]
Citation | Mostafa DIA, El Shamy EN, Mostafa ESA, Rasheed NA (2021). The use of biological antimycotoxin in amelioration of ochratoxicosis in broiler chicken and decrease of toxin residues in the breast muscles. Adv. Anim. Vet. Sci. 9(3): 320-329.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.3.320.329
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mostafa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Ochratoxins are a family of toxic compounds produced as secondary metabolites of several species, including the fungal genera Aspergillus and Penicillium (Varga et al., 1996; Larsen et al., 2001; Bayman et al., 2002). Ochratoxin A (OTA) is one of the most important and deleterious mycotoxins (Jørgensen, 1998; Santos et al., 2009). It is ubiquitously found all over the world in many foodstuff and reported to be nephrotoxic, hepatotoxic, teratogenic and immunosuppressive (Nayak et al., 2014). Several toxic effects of OTA have been described, such as inhibition of protein synthesis, impairment of calcium homeostasis, induction of lipid peroxidation, oxidative stress, and DNA damage, with species and tissue-specific differences (Andersen and Thrane, 2006). It has been found to cause poultry nephropathy (Malir et al., 2016), and the mechanisms leading to OTA nephrotoxicity, hepatotoxicity, and immunotoxicity can be linked to the inhibition of protein synthesis, lipoperoxidation, and modulation of the MAP kinase cascade.
In poultry, the ingestion of OTA causes lesions in the hematopoietic system, thus leading to the suppression hematopoiesis (Moura et al., 2004). It causes myelotoxicity and immunosuppressive effects on the thymus, bursa of fabricius, spleen, and lymph nodes (Bennett and Klich, 2003). Commission Recommendation, (2016) established 0.1 mg /kg diet as the acceptable value for ochratoxin A in poultry feed
Broilers feed on a diet contaminated with OTA that can impose a risk for humans who consume their meat due to the accumulation of its residues on their muscles and edible offal (Bozzo et al., 2011) and it is heat-stable and has an accumulative effect. Considerable economic implications involved and the hazardous effects of mycotoxin residues in animal products on human health, so considerable attention is needed to develop alternative pathways to counteract the problem of ochratoxicosis (Narote et al., 2020).
In humans, exposure to OTA has been linked with Balkan endemic nephropathy (BEN), a chronic tubule-interstitial disease associated with progressive renal fibrosis and tumours of the renal pelvis and urethra (Pfohl-Leszkowicz, et al., 2002; Pfohl-Leszkowicz, 2009). Furthermore, the Scientific Committee on Food (SCF) in 1998 underlined that OTA possesses carcinogenic, nephrotoxic, teratogenic, immunotoxic and possibly neurotoxic properties (SCF, 1998).
Biological detoxification of mycotoxins by the use of microorganisms is one of the well-known strategies for the management of mycotoxins in foods and feeds. Among the different potentially decontaminating microorganisms, Saccharomyces cerevisiae and lactic acid bacteria represent unique species which are widely used in food fermentation and preservation (Gourama and Bullerman, 1995; He et al., 2010).
Śliżewsk and Piotrowska, (2014) proved that the probiotic preparation containing Lactobacillus strains and Saccharomyces cerevisiae were characterized as substances with the highest capability of ochratoxin biodegradation. The application of probiotic cultures of bacteria and yeasts inhibits the growth of molds, as well as the production of mycotoxins (Stidl et al., 2007). Bacillus subtilis is considered to be an important biocontrol agent against several toxin-producing pathogenic fungal strains and Generally Recognized As Safe (GRAS) (Thakaew and Niamsup 2013, Piotrowska M., 2014, Shukla et al., 2018). Probiotics produce enzymes that decompose carbohydrates. They also increase the activity of the host’s enzymes, such as β-galactosidases, saccharase, and maltase. Vitamin C and vitamin E also have beneficial effects in reversing the negative effects of OTA (Haazeleel et al., 1993; Tous et al., 2003).
Based on this information, we aimed in this study to explore the effects of OTA on broilers by detecting the clinical signs, postmortem lesions, hematological, immunological changes and kidney function tests and determine the its residues in the breast muscle of the broilers with a trial to eliminate their harmful effects and residues and by using biological anti-mycotoxin.
Materials and Methods
Birds
The study was conducted in the Animal Health Research Institute, Zagazig, Sharkia, Egypt. The protocol was approved according to the National Regulations on Institutional Animal Care and Use Ethical Committee and Animal Welfare (75429/27-8-2020). Sixty, one day old, Hubbard broiler chicks were used for this experiment. The birds, had free access to both feed and water, and a 22-light, 2-dark lighting regimen was used during the experimental period. During the first week of age, the temperature was kept at 34 °C, and then reduced by 3°C / week until the broiler chicks were 4 weeks-old. The experimental diets were formulated to offer all the nutritional considerations for chicks during different periods of growth (AVIAGEN, 2014) and tested for being free from OTA using OTA ELISA test kits from Shenzhen Lvshyuan Biotechnology Co.
Chemicals
The purified and crystalline forms of OTA were obtained from Sigma Company (St. Louis, MO, USA) and used at a dose of 0.2 mg/kg diet according to (Sakthivelan and Rao, 2010; Aisha et al., 2013; Joo et al., 2013). The purified OTA was added and mixed with the ration according to (Pozzo et al., 2012), then the ration was analyzed to confirm the OTA concentrations using OTA ELISA test kits from Shenzhen Lvshyuan Biotechnology Co.
The biological anti-mycotoxin (Bio-Stress) obtained from Pharma Sweed-Egypt, 10th of Ramdan City-B3 consists of: (Bacillus subtiliss; 5 × 104 colony/gm, vitamin E; 20 mg/gm (20 IU/gm) as dl-α-tocopheryl acetate, selenium as sodium selenite; 360 ug/gm, and vitamin C; 400 mg/gm) and used by 1 gm/liter of drinking water, as recommended by the producing company.
Experimental design
A total of 60 one-day old Hubbard broiler chicks were randomly divided into three experimental groups each group was 20 birds. The first group was kept as control and was fed on ration detected to be free from OTA, the second was fed on ration containing 0.2 mg/kg OTA (Sakthivelan and Rao, 2010; Aisha et al., 2013; Joo et al., 2013), and the last one was fed on ration with OTA with the same dose as group (2) and biological anti-mycotoxin, specifically 1gm/liter of drinking water from the first day till the end of the experiment.
When the appearance of clinical signs of OTA toxicity began (this was at 32 days old) in gp. (2), five birds, following normal distribution, were randomly selected from each group for sampling. The clinical signs and postmortem lesions were recorded.
Sampling
Five birds from each group, following normal distribution, were randomly selected and three blood samples from each bird were collected from the wing vein under sterile condition using sterilized syringes, by the time the symptoms appeared (this was at 32 days old). The first sample (2 ml) was received into a clean heparinized tube containing phagocytic assy. The second sample (1 ml) was transferred into a clean tube containing dipotassium salt of EDTA for hematological examination. The third blood sample was left for 2 hours to clot at room temperature then centrifuged at 3000 rpm for 15 min., and sterile Pasteur pipettes were used to collect the clear serum and it was transferred to labeled, sterile, and dry eppendorf tubes for biochemical analysis.
The tissue samples: a portion of breast muscle were obtained immediately after sacrificing the birds from all groups, and they were kept frozen for OTA residue analysis.
Hematological studies
Erythrocytic and leukocytic counts: Erythrocyte counts were recorded using an improved Neubauer hemocytometer and Natt and Herrick solution as a special diluent for chicken blood as described by (Harrison and Harrison 1986). The packed cell volume was estimated using micro hematocrit centrifuge as described by (Coles, 1986) Hemoglobin level was estimated using the cyanomethemoglobin colorimetric method after centrifugation as previously described by (Van Kampen and Zijlstra, 1983). Mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) were calculated. Blood films were prepared, fixed using methyl alcohol, and stained by Giemsa for estimating the differential leukocyte count as described by (Feldman et al., 2000).
Immunological studies
The measurment of phagocytic activity of peripheral blood monocytes using Candida albicans was adapted as described by (Antony et al., 1985; Chu and Dietert, 1989).
2.6.2. The serum lysozyme concentration was determined using a lysozyme kit (Chicken lysozyme/Muramidaes ELISA kit) of CUSABIO, Indomedix, as described by (Wu et al., 2007).
Biochemical studies
The serum total protein level was colometrically determined as previously described by (Krohn, 2005), the serum albumin and immunoglobulin were done by agarose gel electrophoresis as previously described by (Henery et al., 1974) and the level of serum globulin was determined via subtracting the serum albumin from the total protein. The A/G ratio for each sample was estimated using the value of albumin and globulin. Serum uric acid, calcium, and inorganic phosphorus were determined colometrically using diagnostic kits as previously described by (Tietz and al., 1995), by test kits of Diamond-Egypt for serum uric acid, calcium and of Spectrum for phosphorus.
Detection of OTA residue
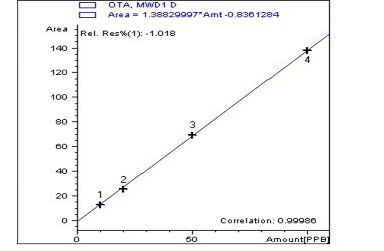
Five tissue samples (breast muscles) from each group were prepared for the detection of OTA residues according to (AOAC, 2000) using the (HPLC) Agilent Series 1200, using a fluorescence detector (FLD) with excitation at 360nm and emission at 440 nm at Animal Health Research Inistiute, Dokki, Giza. The prepared samples were examined individually for the determination of ochratoxin A levels by a validated method previously recorded by (Toscani et al., 2007).
Statistical analysis
The data obtained from this investigation were statistically analyzed by F-test according to (Tamhane and Dunlop, 2000) using the MSTAT-C computer program. The treatment methodss were compared by the least significant difference test at p ≤ 0.05 statistical level. The means in the same column that are followed by different letters were statistically significant and the highest values were represented with the letter (a) and Data were presented as mean ± SE.
Results
Clinical signs and postmortem lesions
At 32 days-old, the birds from group 2 showed diarrhea, reducted feed intake, thirst, restlessness, dullness, enlarged abdomen, emaciation, and ruffled feathers (Fig. 1). Postmortem examination, revealed enlargement and congestion in internal organs, hemorrhage, friable liver, distended bladder, and decreased sizes of the thymus and bursa of (Fig. 2). The birds of group 1 and 3 showed normal appearance.
Table 1: The effects of OTA and anti-mycotoxin on several hematological and immunologic parameters (Mean ± SE) in different groups (n = 5).
| Parameters | Control | OTA | OTA & anti-mycotoxin |
|
RBCs count (×106cells /μl) |
2.7 ± 0.09a |
2.22 ± 0.01b |
2.62 ± 0.06a |
| Hemoglobin (gm%) |
9.63 ± 0.44a |
8.2 ± 0.1b |
8.98±.0.25a |
| PCV % |
34.5 ± 1.81a |
29.63 ± 0.13b |
33.1 ± 0.4 a |
| MCV fl |
127.57 ± 2.65b |
133.5 ± 0.001a |
126.53 ± 2.59b |
| MCHC % | 27.93 ± 0.23 | 27.7 ± 0.20 | 27.47 ± 0.13 |
|
TLC count (×103cells /μl) |
38.33 ± 2.96a |
31.62 ± 0.67b |
36.93 ± 1.5a |
|
Lymphocyte (×103cells/μl) |
22.11 ± 2.72a |
16.8 ± 0.93b |
18.43 ± 0.52ab |
|
Heterophil (×103cells /μl) |
12.27 ± 0.68ab |
10.88 ± 0.76b |
14.76 ± 0.92a |
|
Monocyte (×103cells /μl) |
0.66 ± 0.11b |
1.03 ± 0.17a |
0.67 ± 0.1b |
|
Esionophil (×103cells /μl) |
1.79 ± 0.17 | 1.59 ± 0.30 | 1.7 ± 0.04 |
|
Basophil (×103cells /μl) |
1.5 ± 0.03 | 1.41 ± 0.10 | 1.37 ± 0.12 |
| Phagocytic % |
64 ± 2.3b |
52 ± 2.3c |
75 ± 5.2a |
| Phagocytic index |
3.25 ± 0.23b |
2.62 ± 0.01c |
3.88 ± 0.03a |
|
Lysozyme (μg/ml) |
0.19 ± 0.005a |
0.14 ± 0.007b |
0.17 ± 0.013ab |
Values in each row that are followed by different letters are significantly different at p ≤ 0.05.
n: number of samples; OTA: Ochratoxin A; RBCs: Red blood cell count; PCV: Packed cell volume; TLC: Total leukocytic count; MCV: Mean corpuscular volume.; MCHC: Mean corpuscular hemoglobin concentration
Table 2: The effects of OTA and anti-mycotoxin on some biochemical parameters (Mean ± SE) in different groups (n = 5).
| Parameters | Control | OTA | OTA & anti-mycotoxin |
| Total protein (g/dl) |
3.31 ± 0.22a |
2.07 ± 0.14b |
3.62 ± 0.06a |
| Albumin (g/dl) |
2.00 ± 0.24a |
1.39 ± 0.12b |
2.23 ± 0.12a |
| Globulin (g/dl) |
1.31 ± 0.15a |
0.68 ± 0.02b |
1.39 ± 0.14a |
| A/G ratio |
1.53 ± 0.33b |
2.03 ± 0.11a |
1.63 ± 0.22b |
| alpha1globulin (g/dl) |
0.12 ± 0.08a |
0.06 ± 0.006b |
0.11 ± 0.007a |
| Alpha2globulin (g/dl) |
0.17 ± 0.06a |
0.12 ± 0.058b |
0.18 ± 0.06a |
| Beta globulin (g/dl) |
0.58 ± 0.013a |
0.20 ± 0.006b |
0.67 ± 0.09 a |
| Gamma globulin (g/dl) |
0.45 ± 0.012a |
0.30 ± 0.026b |
0.43 ± 0.011a |
| Creatinine (mg/dl) |
0.41 ± 0.006b |
0.71 ± 0.01a |
0.41 ± 0.05b |
| Uric acid (mg/dl) |
5.0 ± 0.64b |
7.47 ± 0.14a |
5.3 ± 0.32b |
| Phosphrus (mg/dl) |
6.25 ± 0.5b |
7.9±a |
6.16 ± 0.09b |
| Calcium (mg/dl) |
8.37 ± 0.37a |
5.9 ± 0.11b |
8.01 ± 0.05a |
Values in each row that are followed by different letters are significantly different at p ≤ 0.05.
n: number of samples; OTA: Ochratoxin A; A/G: Albumin globulin ratio
Hematological studies
A Significant decrease (p ≤ 0.05) in RBC count, hemoglobin content, and hematocrit value (82.22%, 85.15% and 85.88%) with an increase in the mean corpuscular volume were detected in group 2 compared with the control group (Table 1). The birds in group 3 showed no significant change in RBC count, hemoglobin content, and hematocrit value when compared with the normal control group. Concerning the leukogram, a significant decrease in the total leukocytic count and lymphocytic count, with no significant changes in the heterophil, basophil and eosinophil counts were observed in group 2, and also, there was no significant changes in all examined leukograme parameters in group 3 compared with normal control group.
Immunological studies
A significant decrease (p ≤ 0.05) in phagocytic% (64%), phagocytic index (80.61%), and lysozyme concentration (77.78%) was found in the OTA-treated group, while significant increase was found in group 3 compared with the normal control group (Table 1).

Figure 1: Clinical signs of group (2) broilers treated with OTA. Show restlessness, emaciation, dullness and ruffled feathers
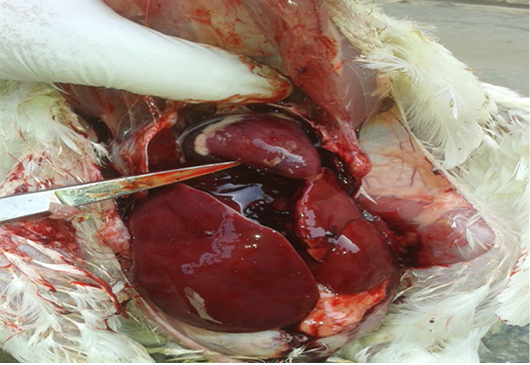
Figure 2: Postmortem lesions of group (2) broilers treated with OTA. Show enlargement and congestion in internal organs, hemorrhage and friable liver.
Biochemical studies
A significant decrease (p ≤ 0.05) in total protein, albumin, globulin, Alpha1, alpha 2 and beta globulin was observed in the birds from group 2, wherase a significant increase in albumin/globulin (A/G) ratio in the OTA-treated group, a significant increase in serum creatinine, uric acid, and phosphorus levels, with a significant decrease in serum calcuim level was observed in group 2, compared with the normal control group (Table 2). No significant change was observed in all examined biochemical parameters in group 3.
Detection of OTA residue in breast muscle
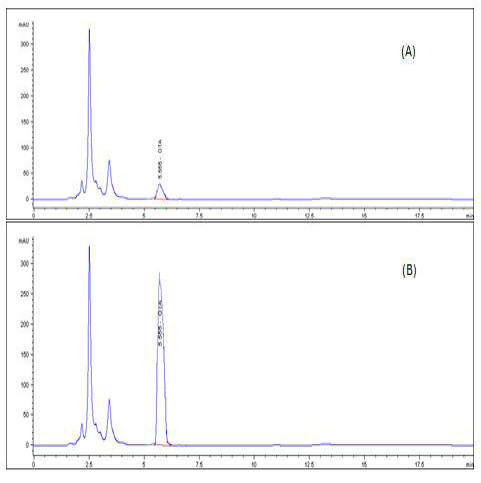
A highly significant increase (p ≤ 0.05) the in level of OTA residue in the breast muscle of the birds from group 2 (153%), when compared with birds of group 3 that were treated with anti-mycotoxin and the control group that had ration free from OTA (Table 3) and (Figures 3–5).
Table 3: The effects of OTA and anti-mycotoxin on OTA residues on the breast muscles (Mean ± SE) in different groups (n = 5).
| Parameters | Control | OTA | OTA & anti-mycotoxin |
| OTA residue in breast muscles ppb | - |
153±10.6a |
6.45±0.82b |
Values in each row that are followed by different letters are significantly different at p ≤ 0.05.
n: number of samples; OTA: Ochratoxin A
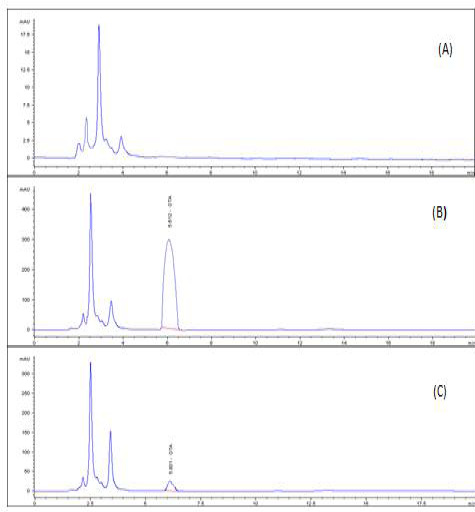
Figure 5: HPLC chromatogram of OTA residues in breast muscle sample (A) Control muscle sample (B) Positive OTA muscle sample (C) Positive OTA- and anti-mycotoxin-treated muscle sample.
Discussion
The development of effective strategies alleviating OTA-induced toxicity is very complex because the mechanism of action of OTA is still unclear. The toxic effects are manifested as the inhibition of protein synthesis, lipoperoxidation, direct genotoxicity, and cell cycle arrest (Kőszegi and Poór, 2016).
Macrocytic normochromic anemia, represented by a significant decrease in RBCs count, Hemoglobin content, and hematocrit value were observed in the OTA-treated group, with a marked increase in mean corpuscular volume. This was in agreement with Bennett and Klich, (2003); Elaroussi et al. (2006); Naik et al. (2017), who previously mentioned that feeding chickens with OTA may promote anemia by reducing concentrations of red blood cells and hemoglobin. Anaemia developed in ochratoxin fed birds might be as a result of depressed erythropoiesis due to nephropathy and defective protein metabolism due to hepatopathy (Meshram et al., 2017), in addition, this reduction in Hb concentration may be attributed to the reduced protein synthesis, inhibition of protein synthesis (Sawarkar et al., 2012) and impaired iron absorption combined with suppression of haemopoiesis (Huff et al., 1975) also Moura et al. (2004) previously reported that in birds, the ingestion of OTA causes lesions in the hematopioetic system, and hematopoiesis is suppressed. This was in contrast to Tansakul et al. (2012) and Pozzo et al. (2013), who previously revealed that feeding broiler chickens a diet contaminated with 0.1 mg OTA/kg, which was the maximum level admitted by the European Commission Recommendation, did not affect the hematological parameters this was due the low dose used.
Our results in group 3 revealed insignificant changes in RBC count, hemoglobin content, and hematocrit value, when compared with the normal control group, as the probiotics and vitamins in the antimycotoxin-containing feed improved the protein biosynthesis. They also increase the activity of the host’s enzymes (Haazeleel et al., 1993; Tous et al., 2003).
The evaluation of the immune status of the examined birds revealed a significant decrease in the total leukocytic count and lymphocytic count, phagocytic %, lysozyme concentration, and gamma globulin in OTA-treated group, which agreed with Nayak et al. (2014), who previously reported a significant decrease in the total leukocytic count of broilers fed with ration containing 2 ppm of OTA for 35 days. Singh et al. (1990) previously reported a decrease in lymphocytic count and phagocytic activity of the splenic macrophages of broilers that were fed with 0.5 ppm and 2 ppm OTA on the 21st day of intoxication. The decrease in phagocytic % and index in this study may be attributed to the impairment of the reticuloendothelial functions that is induced by ochratoxicosis (Zul-Hassan et al., 2012). Muller et al. (1995) previously stated that OTA has a non-selective suppressive effect on various immune and defense reaction. Rupœ et al. (1978) formerly recorded a reduced content of gamma globulin. On another note, Awaad et al. (2001) earlier reported no significant change in phagocytic %, phagocytic index, and lysozyme concentration of one-day old chickens that were fed with 50 μg/kg of OTA for 3 and 5 weeks which may be due the low OTA dose used. The monocytic count demonstrated a significant increase in the OTA-treated group, compared with other groups. The findings agreed with the results previously stated by Moura et al. (2004), who attributed that to the suppression in hematopoiesis caused by the OTA toxin and its myelotoxicity and immunosuppressive effects (Huff et al., 1988; Bennett and Klich, 2003).
The improvement in the immunologic status of the birds from group 3, when compared with group 2, was represented by normal total leukocytic count, lymphocytic count, heterophil count, phagocytic%, phagocytic index, and lysozyme concentration. This may be due to the vitamins and Bacillus subtiliss bacteria in the anti-mycotoxin (Tous et al., 2003; Shukla et al., 2018).
The total protein, albumin, globulin, A/G ratio, alpha1globulin, alpha2 globulin, and beta globulin were significantly decreased in the OTA-treated group than the other groups, and these results agreed with Anand et al. (2018); Meshram et al. (2017); Naik et al. (2017); Sakthivelan and Rao, (2010); Singh and Mandal (2018) and Pozzo et al. (2013), who previously revealed that OTA had an overall immunosuppressant effect, which causes a reduction in the total serum protein, albumin, alpha, beta, and gamma globulin concentration. Huff et al. (1975) earlier reported that the total protein and albumin were the sensitive indicators of ochratoxicosis. The mechanism by which OTA produces hypoproteinaemia and hypoalbuminaemia is due to inhibition of phenylalanyl transfer-RNA-synthetase with phenylalanine, which inhibits protein biosynthesis (Hamilton et al., 1977; Konrad and Roeschenthaler, 1977) and renal leakage of albumin resulting from the kidney lesions induced by OTA (Huff et al., 1975). Moreover, OTA has a strong negative effect on cellular energy (ATP) production (Poór et al., 2014). Mitochondrial dysfunction is an early sign of toxicity (Aleo et al., 1991), which results in an overall decrease in protein synthesis. OTA also causes myelotoxicity and immunosuppressive effects on the bursa of , spleen, and lymph nodes, as described by Bennett and Klich, (2003).
This was eliminated in the anti-mycotoxin group 3, by enhancing the protein biosynthesis, so no significant change was observed in the total protein, albumin, globulin, and A/G ratio of this group. The same was observed with alpha1globulin, alpha2 globulin, and beta globulin because the probiotics and vitamins in the antimycotoxin-containing feed elemenate the adverse effect of the OTA on the immunologic status of the birds and improved the digestion and usage of these feeds by producing the hydrolytic enzymes that decompose carbohydrates. They also increased the activity of the host’s enzymes, such as β-galactosidases, saccharase, and maltase (Ŝtyriak et al., 2001).
Serum creatinine, uric acid, and inorganic phosphorus levels showed a significant increase, whereas serum calcium levels significantly decreased in the OTA-treated group. These were previously documented by Huff et al. (1988); Sreemannarayana et al. (1989); Elaroussi et al. (2008); Singh and Mandal (2018), who noted that chicken that were fed with high levels of OTA contaminants resulted in a significant increase in serum uric acid and, but it is in contrast to Tansakul et al. (2012), who previously observed that OTA contamination had no effect on blood chemistry profiles, as the specific indicators of OTA exposure did not increase when OTA was administered to ducks (15 days-old) through their feed that was contaminated with low doses of OTA.
OTA acts essentially in the proximal renal tubules, inhibiting the enzyme phosphoenolpyruvate carboxylase, which is a lipid peroxidant that alters the structural and functional renal ability to mobilize calcium (Betina, 1989). The insignificant changes in serum (this was at 32 days old) , uric acid, calcium, and serum phosphorus level in birds of group 3 may be due to the use of anti-mycotoxin, which eliminates the nephrotoxic effect of the OTA on the kidney.
We detected high level of OTA residue in the breast muscle of group 2 treated with OTA, as compared with the control group and group 3. The studied result agreed with Zaghini et al. (2007), who formerly found OTA residues in the kidney, muscle, and blood of laying hens. This was in contrast with Pozzo et al. (2013) who previously repoted that the maximum tolerable level of 0.1 mg OTA/kg, established for poultry feeds by the EU represents a safe limit for the final consumer, because no OTA residue was found in breast and thigh meat. The OTA accumulation in chicken tissues is due to its long half-life (4.1 h) (Galtier et al., 1981), since after the its absorption in the gastrointestinal tract, it binds primarily to albumin with high affinity, which results in its very long half-life (from a few days to one month, depending on the species) (Kőszegi and Poór, 2016).
In the present study, the use of biological anti-mycotoxin, which contained Bacillus subtilis, vitamin E, selenium, and vitamin C, at a dose of 1 gm/liter of drinking water had overcome the toxic effects of OTA on the broilers and improved the hematological, immunological, and biochemical parameters of the bird. Moreover, this significantly decreased the toxin residue in the breast muscles. The biological detoxification of mycotoxins by the use of microorganisms is one of the well-known strategies for the management of mycotoxins in foods and feeds. These bacteria inhibit the growth of molds, as well as the production of mycotoxins (Ŝtyriak et al., 2001; Fuchs et al., 2007).
Conclusion, the present study was demonstrated the harmful effects of OTA on the broilers, which was manifested as anemia represented by significant decrease in RBCs count, Hb and PCV, immunosuppression represented by significant deviation in the leukogram, protinogram, lysozyme activity, phagocytic% and ratio in addition to nephrotoxicity represented by significant alteration in renal function tests. The researchers also explained the role of the biological anti-mycotoxin in the elimination of these deleterious effects and improvement of the hematological, biochemical, and immunologic parameters. The detection of the high levels of OTA in the muscle tissue of the chickens makes it unsuitable for human consumption, as the toxin is highly heat-stable and its complete removal from human food is practically impossible. The use of anti-mycotoxin significantly decreases the toxin residues in the breast muscle.
Conflict of Interest
All authors declare that there is no conflict of interest.
authors contribution
All authors sharing in the project design and performed the hematological, immunological and biochemical investigations, and data analysis equally. They are all sharing in the preparation of the manuscript (writing and revision) and approved the final version of it before publication.
References