Advances in Animal and Veterinary Sciences
Research Article
Inhibited TLR-4/NF- κB Pathway Mediated by Cannabinoid Receptor 2 Activation Curbs Ongoing Liver Fibrosis in Bile Duct Ligated Rats
Alaa Mohamed Ali1, Osama Samir El-Tawil2, Sahar Samir Abd El-Rahman1
1Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Egypt; 2Department of Toxicology, Forensic Medicine and Veterinary Regulations, Faculty of Veterinary Medicine, Cairo University, Egypt.
Abstract | The endocannabinoid system is participated in the pathogenesis of liver fibrosis. Activation of cannabinoid receptor type 2 (CB2) plays a principle role in liver regeneration and protection against liver injury. This study aimed to explore the role of CB2 agonist (AM1241) on suppressing the progress of liver fibrosis induced by bile duct ligation (BDL). AM1241 was administrated to BDL rats in two doses (3 and 6 mg/kg). Liver function and oxidative stress markers, hepatic TNF-α, IL-6 and IL-10, RT-qPCR expression of TLR4 gene, immunohistochemical expression of CD31, CD34, α-SMA and NF-κB as well as histopathology of liver tissue were all assessed. BDL rats (n=9) showed; significant (P<0.05) increase in aminotransferases activity, bilirubin levels, hepatic MDA, TNF-α and IL-6 levels and TLR4 gene-expression with significant decrease in GSH content and IL-10, marked histological fibrosis, pseudolobulation and cholangiolar proliferation with increased immune-expression of CD31, α-SMA and NF-κB with nil expression of CD34. Conversely, AM1241 administration at both doses significantly (P<0.05) ameliorated liver function markers, MDA level, pro-inflammatory cytokines TNF-α and IL-6 and TLR4 gene expression. Additionally, AM1241 significantly (P<0.05) increased GSH content and immunomodulatory cytokine; IL-10. Histologically, AM1241 limited fibroplasia extension, decreased cholongiocytes proliferation, and decreased immune-expression of CD31, α-SMA and NF-κB with strongly expressed CD34. The current study depicts that; CB2 receptors activation promotes liver regeneration and suppresses the progress of liver fibrosis through featured mechanisms including; TLR4/NF-κB pathway-dependent inhibition, suppressing pro-inflammatory cytokines; IL-6 and TNF-α, reducing angiogenesis and stimulating hepatic oval/progenitor cells.
Keywords | Cannabinoid receptor type 2, Liver fibrosis, TLR4, NF-κB, CD31
Received | October 12, 2020; Accepted | November 03, 2020; Published | January 01, 2021
*Correspondence | Sahar Samir Abd El-Rahman, Prof. of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt; Email: [email protected], [email protected]
Citation | Ali AM, El-Tawil OS, El-Rahman SSA (2021). Inhibited TLR-4/NF- κB pathway mediated by cannabinoid receptor 2 activation curbs ongoing liver fibrosis in bile duct ligated rats. Adv. Anim. Vet. Sci. 9(2): 253-264.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.2.253.264
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Ali et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Hepatic fibrosis is a dynamic process that results from injury of liver tissue. formerly, it was ratified that liver fibrosis is irreversible, however, recent data allows us to believe that fibrosis is an active possible reversible process originating from a response to wound-healing of chronic liver injury (Parfieniu and Flisiak, 2008). It is scientifically accepted that there are many reasons for liver damage, which when prolonged, liver fibrosis results. As for example; hepatotropic viruses infections such as hepatitis B and C viruses (HCV), chronic exposition to various drugs or toxins such as alcohol abuse, cholestatic diseases and chronic autoimmune hepatitis (Hernández-Aquino et al., 2017). The mechanism triggering the occurrence of hepatic fibrosis is the imbalance between the deposition and the removal of extracellular matrix (ECM). The key fibrogenic cell in liver tissue is the hepatic stellate cells (HSCs), which are the dominant producers of ECM. The activation of HSCs and their proliferation are mediated by various cytokines during the progress of liver injury (Ahmad et al., 2014). Bile duct ligation is the most common technique for induction of animal model of cholestatic liver injury as reported by (Aller et al., 2008).
Silymarin is a milk thistle extract which consists of a complex mixture of one flavonoid and seven main flavonolignans. The hepatoprotective and therapeutic effects of silymarin being due to its phytoconstituents which have powerful anti-inflammatory and antioxidant properties (Mastron et al., 2015). Cannabinoid receptors are part of the endocannabinoid system which is implicated in many physiological processes including; mood, appetite, sensation of pain, and memory. It consists mainly of two types of G-protein-coupled receptors, cannabinoid CB1 and CB2 receptors (Pertwee, 2005). They are well-known to be involved in cell proliferation and inflammatory responses. In normal liver, cannabinoid receptors expression is very low, partially because they are not fully expressed in hepatocytes. However, their expression was found to be up-regulated in vascular endothelial cells and hepatic myofibroblasts along the course of chronic progressive liver disease (Teixeira-Clerc et al., 2006).
Although CB1 receptor is notarize to have a profibrogenic effect in liver, CB2 receptors have confirmed to have an antifibrogenic activity (Julien et al., 2005).
CB1 receptors expression was found to be increased in cirrhotic liver specimens of human particularly in HSCs when they are transformed into myofibroblasts in chronic liver injury (Teixeira-clerc et al., 2006). In 2005, agonists of cannabinoid receptors were licensed in Canada in advanced cancer patients as an adjunctive analgesic treatment as well as in multiple sclerosis cases to relief the neuropathic pain. Additionally, in 2010, cannabinoid receptor agonists were licensed in the UK and Canada for the spasticity treatment which observed as a result to multiple sclerosis and have more recently they have approved as a medicine in several other countries.
Hence our aim in the current work was to clarify the mechanism of the antifibrotic effects of synthetic CB2 agonists; AM1241 on the ongoing liver fibrosis in bile duct ligated rats, by studying its suppressive effect on specific TLR4/NF-κB pathway and angiogenesis. Also we tried to explain ability of CB2 activation by AM1241 to regenerate liver tissue by tracing its effect on stimulating progenitor cells. Finally, we studied whether the aforementioned effect will be dose-related when increase the dose or not.
MATERIALS AND METHODS
Chemicals
AM1241 was purchased from (Abcam, USA, ab120934). It was dissolved in DMSO (Sigma-Aldrich, USA, 03310) 10 mg/ml (Drug-DMSO), then a daily aqueous buffer of the drug was prepared by diluting one part of the drug-DMSO mixture in two parts of PBS at doses of 3 and 6 mg/kg b.w/ i.p. Silymarin was purchased from (Sigma-Aldrich, USA, S0292) and was administrated orally at a dose of 100 mg/Kg/day. Ketamine (Sigma-Aldrich, USA, K2753), was used in a dose of 50 mg/kg/i.p (Struck et al., 2011). Xylazine (ADWIA, Egypt, 2002271), was used in a dose of 5 mg/kg/i.p (Struck et al., 2011). Ceftriaxone (EPICO, Egypt, 1807561A), was used in a dose of 30 mg/kg/i.m (Aller et al., 2008).
Experimental animals
Forty-five adult male wistar rats, weighing 180–200g were utilized in the present work; they were obtained from CLAVCAP-VACSER, Cairo, Egypt. Rats were housed in plastic cages under managed conditions, they were given a standard commercial pellet diet (containing at the very least 5% fiber, 20% protein, 3.5% fat, 6.5% vitamins and ash mixture) and offered water ad libitum. Rats were kept all over the experimental period under good hygienic conditions and they were left one week for acclimatization on lab conditions; 12:12 light: Dark cycle, 25 ± 2 °C and 50% ± 20% relative humidity before onset of the experiment.
Ethical statement: All investigations and experiments utilizing animals were performed according to the protocol endorsed by the Institutional Animal Care and Use Committee at Cairo University, IACUC (CU II F 26 19).
Experimental design
Randomly, rats were divided into 5 groups, 9 rats each. Group 1; sham operated group; rats exposed to surgery without bile duct ligation and served as control non-treated, they received i.p. injection of 0.1 ml of 1:2 solutions of DMSO: PBS (vehicle). Group 2; bile duct ligated group; rats exposed to bile duct ligation surgery (Georgiev et al., 2008) then they received vehicle as group 1. Group 3; bile duct ligated rats received the standard drug, 100 mg/Kg/day silymarin by gavage (Mahli et al., 2015). Groups 4 and 5; bile duct ligated rats were i.p injected with the investigated drug; AM1241 at a dose of 3 and 6 mg/kg (Barutta et al., 2011) respectively.
All treatments started two weeks post-surgery (post fibrosis induction) and continued for two weeks after induction of fibrosis (Mahmoud et al., 2014).
Blood and tissue samples
Toward the end of the experimental period, blood samples were gathered from the retro-orbital venous plexus under anesthesia using ketamine (Sigma-Aldrich, USA) 50 mg/kg. i.p. following this rats were authorized by cervical dislocation. Liver was dissected out, wiped of blood and each liver was cut into two sections; one was kept in 10% neutral buffer formalin and the other one was kept frozen at −80°C until further investigations.
Biochemical analysis
The activities of alanine aminotransferase (ALT) (Sigma-Aldrich, USA, 10105589001), aspartate aminotransferase (AST) (Sigma-Aldrich, USA, MAK055), Alkaline phosphatase (ALP) (Biodiagnostic, Egypt, AP1020), total and direct bilirubin (Sigma-Aldrich, USA, MAK126) were all estimated in serum samples of rats of all groups.
Preparation of hepatic tissue homogenate
From each hepatic tissue, 0.5gm was homogenized with ice-cooled saline utilizing a homogenizer (Medical instruments, MPW-120, Poland), to get 20% w/v homogenate. The obtained homogenate was subjected to centrifugation in a cooling axis (Laborzentrifugen, 2k15, Sigma, Germany) at 4000 rpm for 5 min at 4°C to get rid of cell debris. The gained aliquot was kept at −80°C for additional biochemical investigation.
Tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and interleukin-10 (IL-10) were all determined in homogenate using ELISA kits (Cusabio Biotech®, CSB-E11987r, CSB-E04640r and CSB-E04595r respectively, Germany) according to the manufacturing instructions. Reduced glutathione content (GSH) (Ellman, 1959), and malondialdehyde (MDA) level (Ruiz-Larrea et al., 1994) were also determined.
Quantitive real time PCR (RT-qPCR)
Quantitative reverse transcription polymerase chain reaction (RT-PCR) was performed for detecting the expression of TLR4 genes through the following steps.
First RNA extraction: The all-out RNA was secluded utilizing Qiagen tissue extraction Kit (Qiagen, USA) as indicated by directions of manufacture. Second cDNA synthesis: The absolute RNA (0.5–2μg) was utilized for cDNA conversion utilizing high limit cDNA reverse transcription kit (Fermentas, USA). Then Real-time qPCR using SYBR Green I. Real-time qPCR amplification and examination were performed utilizing an Applied Biosystem with software version 3.1 (StepOne™, USA). The Primer sets that were optimized at the annealing temperature are presented in Table 1. The reactions of amplification were carried out in a 50 ml final volume, with thermal cycling conditions of 50 1C for 2 min, 95 1C for 10 min, and 40 cycles of 15 s at 95 1C, 30 s at 60 1C, 30 s at 72 1C, and 10 min at 72 1C. Normalization of the cycle threshold (Ct) data was carried out using βeta actin as a housekeeping gene. Relative gene expression was calculated using the ΔΔCT method. The relative quantitation was calculated according to Applied Bio system software using the equation of; ∆ Ct = Ct gene test – Ct endogenous control.
∆∆Ct = ∆Ct sample1 – ∆Ct calibrator
RQ = Relative quantification = 2-∆∆Ct
The RQ is the fold change compared to the calibrator (untreated sample).
Histopathological and immunohistochemical evaluations
In a routine manner, liver specimens that were fixed in 10% neutral buffered formalin, were dehydrated in graded series of alcohol followed by clearance in xylol and embedding in paraffin. Serially sections of 4–5 μm thickness were obtained from the prepared paraffin blocks followed by their staining with Hematoxylin and Eosin (H and E) and Masson’s Trichrome staining (MTC) (Bancroft and Gamble, 2008). Metavir scoring system (ranged from F0=no fibrosis to F4=cirrhosis) was used for the evaluation of fibrosis (Bedossa et al., 2003).
For immunohistochemical evaluation, paraffin sections were subjected to antigen retrieval via heating in a microwave oven for 25 min at 720 W, then they were incubated overnight at 4°C with one of the following primary antibodies: rabbit monoclonal anti-rat CD34, CD31, α-SMA and NF-κB (Abcam, Cambridge, MA, USA, ab8158, ab7388, ab7817 and ab220803), at dilutions of; 1:50, 1:50, 1:100 and 1:100 respectively. After washing with PBS, sections were incubated for 30 min at room temperature with the corresponding biotinylated secondary antibody at a dilation of 1:200 (Dako Corp.) and streptavidin/ALPaline phosphatase complex at a dilution of 1:200 (Dako Corp.). DAB (Sigma) was used for visualization of the binding sites of antibody, followed by washing with PBS and counterstaining with hematoxylin for 2–3 min. Finally, samples were dehydrated in increasing ethanol solutions, cleared by soaking twice in xylene for 5 min at room temperature, mounted, examined under a high-power light microscope (Hsu et al., 1981). Quantification of the positive brown area of each marker’s expression was carried out by measuring the optical density in 7 high power microscopic fields using image analysis software (Image J, 1.46a, NIH, USA).
Statistical analysis
SPSS 25.0 statistical software was used and all data were presented as means ±SE. One-way analysis of variance (ANOVA) followed by Tukey post-hoc test were used for performing comparisons between multiple groups. Values of P<0.05 were considered statistically significant. Kruskal wallis H test was used for comparing the frequency data for nonparametric analysis followed by the Mann-Whitney U test. The nonparametric data were presented as median.
Results
Activation of CB2 receptors by synthetic analog (AM1241) ameliorated liver function markers
Bile duct ligated (BDL) rats revealed remarkable liver injury denoted by significant (P < 0.05) increased serum levels of AST, ALT, ALP, direct and total bilirubin when compared to sham operated group (Table 2). Silymarin treated rats showed improved liver function elucidated by significant decreased serum levels of AST, ALT, ALP, direct and total bilirubin compared with that of BDL rats. However, the administration of CB2 agonist (AM1241) to BDL rats significantly (P < 0.05) improved various liver function markers as indicated by significant dose related improvement in serum levels of AST, ALT, ALP, direct and total bilirubin compared to the corresponding silymarin administrated group and BDL group.
AM1241 improved oxidative stress markers (GSH AND MDA), TNF-α, IL-6 AND IL-10 IN hepatic tissue homogenate
Results in (Figure 1) displayed that; BDL rats revealed significant (P < 0.05) increase in MDA level and significant decrease of hepatic GSH content compared with sham operated group. On the other hand; BDL rats treated with silymarin showed significant (P < 0.05) decrease in hepatic levels of MDA and increase in hepatic GSH content compared to BDL rats. The administration of AM1241 (3 and 6mg/kg) for 2 weeks showed significant improvement in hepatic oxidative stress indicated by significant (P < 0.05) decrease in hepatic MDA level and significantly counteracted the increase in hepatic GSH content compared to silymarin administrated and BDL groups. The higher dose of AM1241 induced more significant (P< 0.05) improvement in the oxidative stress markers.
Bile duct ligation resulted in significant elevation (P < 0.05) in hepatic TNF-α, IL-6 and significant decrease in IL-10 levels compared to the shame operated group. While, silymarin administrated group revealed significant (P < 0.05) decrease in hepatic TNF-α and IL-6 with a significant increase in hepatic IL-10 contents as compared to BDL rats. Whereas, AM1241 administration significantly limited the elevation of TNF-α and IL-6 levels and up-regulated IL-10 level as compared to silymarin administrated and BDL groups. More significant amendment in the previous cytokines levels was observed on using the higher dose of AM1241.
AM1241 dowen-regulated the expression of TLR4 gene
Significant up-regulation of gene expression of TLR4 was observed in BDL rats compared to the sham operated group by 5 folds. It was noticed that; silymarin treatment significantly diminished the expression of TLR4 gene compared with BDL group by 2 folds. On the contrary, AM1241 administration to BDL rats’ significantly down-regulated TLR4 gene expression in a dose related manner compared with BDL group by 5 folds and silymarin administrated group by 3 folds (Table 3).
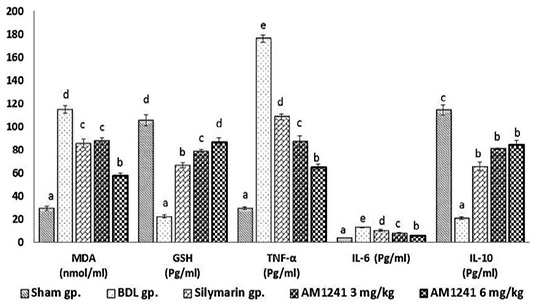
Figure 1: Effect of CB2- activator AM1241 on hepatic tissue homogenate of oxidative stress markers, TNF-α, IL-6 and IL-10 in bile duct ligation model. N=9; Values are expressed as mean ± SE. Data were analyzed by using one-way ANOVA followed by Tukey post-hoc test, a, b, c, d and e are significant at P < 0.05.
Cannabinoid receptor 2 activation by Am1241 considerably inhibited the ongoing liver fibrosis in bile duct ligated rats
Microscopic examination of liver of control sham operated rats’ revealed normal histological structure (Figure 2a). While, examination of liver of fibrosis model rats’ (bile duct ligated rats) showed marked histological alterations. The portal areas in livers of those rats showed congestion of portal vessels, mononuclear inflammatory cells infiltration and marked proliferation of the bile duct epithelium that extended peripherally with many newly formed bile ductuls (Figure 2b). The proliferated cholangiocytes were variable in size and shape, haphazardly insinuating among the hepatic parenchymal cells accompanied with proliferated oval cells (Figure 2c) and sometimes escorted with fibroplasia and mononuclear inflammatory cells. Hepatic cells showed swelling, degeneration, scattered necrosis and apoptosis. Generally, livers of those rats showed distortion of hepatic architecture with the percolated proliferated cells and fibroplasia accompanying with the appearance of many apoptotic cells in the vicinity (Figure 2d). Marked pseudolobulation of the hepatic parenchyma was an obvious finding (Figure 2e), those pseudo lobules were surrounded by thick fibrous septa containing mononuclear inflammatory cells, proliferated bile duct epithelial cells and blood capillaries. Liver of fibrotic rats that treated with silymarin showed mild portal fibroplasia, few inflammatory
Table 1: The oligonucleotide sequences of forward and reverse primers.
| TLR4 |
Forward primer 5′-AATCCCTGCATAGAGGTACTTCCTAAT-3′ Reverse primer 5′-CTCAGATCTAGGTTCTTGGTTGAATAAG-3′ |
|
βeta actin |
Forward primer 5′-TGTTTGAGACCTTCAACACC-3′ Reverse primer 5′-CGCTCATTGCCGATAGTGAT-3′ |
Table 2: Effect of CB2-activatior (AM1241) on serum biochemical markers in bile duct ligation model.
| Groups | AST (U/L) | ALT (U/L) | ALP (U/L) | Total bilirubin (mg/dl) | Direct bilirubin (mg/dl) |
| Sham operated group |
30.000±1.527 a |
27.33 ±1.201 a |
48.66 ±2.027 a |
0.8167±0.742 a |
0.230 ±0.023 a |
| BDL group |
181.00 ±4.35 e |
152.67 ±3.71 e |
206.0 ± 2.34 d |
29.1 ± 0.923 e |
14.233±0.785 e |
| Silymarin group |
74.67 ±3.48 d |
58.67 ± 2.33 d |
94.69 ± 3.84 c |
18.67 ± 0.88 d |
8.51 ± 0.286 d |
| AM1241 (3mg/kg) |
65.30 ± 1.45 c |
48.67± 0.88 c |
76.33 ± 3.5 b |
14.00 ± 1.73 c |
6.05 ± 0.542 c |
| AM1241 (6mg/kg) |
53.66 ± 2.33 b |
35.6 ± 3.17 b |
65.00 ± 2.08 b |
8.05 ± 1.31 b |
4.266 ±0.384 b |
Mean ± SE with different letters (a, b, c, d and e) within the same column are significantly different at P < 0.05. N=9 by One-way ANOVA and followed by Tukey post-hoc test.
Table 3: The relative gene expression of TLR4 in sham group and other treated groups.
| Sham operated group | BDL group | Silymarin administrated group | AM1241 (3 mg/kg) administrated group | AM1241 (6 mg/kg) administrated group |
|
1.0634 ± 0.014a |
6.1615 ±0.402d |
3.9705 ± 0.426 c |
1.5537 ± 0.210b |
1.1548 ± 0.312a |
Values are relative to βeta actin, the house keeping gene. Mean ± SE with different letters (a, b, c, d and e) are significantly different at P < 0.05. N= 9 by one-way ANOVA and followed by Tukey post-hoc test.
Table 4: Correspondence between area of fibrosis intensity, as assessed by image analysis in whole sections and the Metavir scoring.
| Groups | METAVIR score (median) | Area of Fibrosis intensity (Means ± SE) |
| Sham operated group | 0 | 0.1200 |
| BDL group | 5 | 6.1260 |
| Silymarin administrated group | 3 | 4.5340 |
| AM1241 3mg/Kg | 2 | 2.6920 |
| AM1241 6 mg/Kg | 1 | 1.2000 |
cells infiltration and deceased intensity of bile duct epithelium proliferation with appearance of multiple newly formed bile ductuls which were mostly limited to portal areas and mildly extended in the vicinity (Figure 2f and g). While treatment of model rats with AM1241 showed a dose related marked decreased cholangiolar epithelial proliferation, absence of fibroplasia which were both mildly limited to portal areas without any evidence of peripheral extension with an explicit lobular regularity preservation (Figures 2h and 3a-b). Metavir scoring system (ranged from F0=no fibrosis to F4=cirrhosis) was used for evaluating fibrosis extension (Figure 2c). Masson’s Trichrome staining revealed that; the ligation of bile duct markedly resulted in an augmentation in hepatic fibrous components with portal fibrosis (Figure 2c and e). Per contra, silymarin administration to BDL rats significantly reduced fibrosis (Figure 3f) but in a less manner than that observed by AM1241 administration. AM1241 treatment decreased fibrosis compared to the non-treated BDL group (Figure 3g and h). The intensity of fibrous proliferation in different groups is presented in (Figure 3i). Results of histomorphometric analysis of Masson Trichrome staining matched the results of Metavir scoring (Table 4).
Cannabinoid receptor2 activation by AM1241 decreased expression of α-sma, CD31 and NF-κb and increased expression of CD34
Liver of rats of sham operated group showed normal α-SMA immune-expression (Figure 4a) in portal areas and around central veins with negative expression of CD31 (Figure 5a), NF-κB (Figure 6a) and CD34 (Figure 7a). While, livers of BDL rats revealed; marked increased expression of; α-SMA (Figure 4b and c) along the fibrous tissue proliferation, increased CD31 immune-reactive cells (Figure 5b) among the endothelial cells linings hepatic sinusoids as well as increased expressions of NF-κB (Figure 6b and c) in the parenchymal cells together with mild immunopositivity of CD34 (Figure 7b). Livers of silymarin treated rats showed decreased expression of α-SMA (Figure 4d), CD31 (Figure 5c) and NF-κB (Figure 6d), accompanied with increased immune positivity of CD34 (Figure 7c). On the other hand, AM1241 administration significantly limited the expression of α-SMA (Figure 4e and f), CD31 (Figure 5d and e) and NF-κB (Figure 6e and f), but markedly increased the CD34 immune-positive cells (Figure 7d and e), that all was a dose related response with increase the dose of AM2141. The area percent of the positive immune-expression of the four markers (brown color) displayed as optical density in control and all treated groups is presented in Figures 4g, 5f, 6g and 7f.
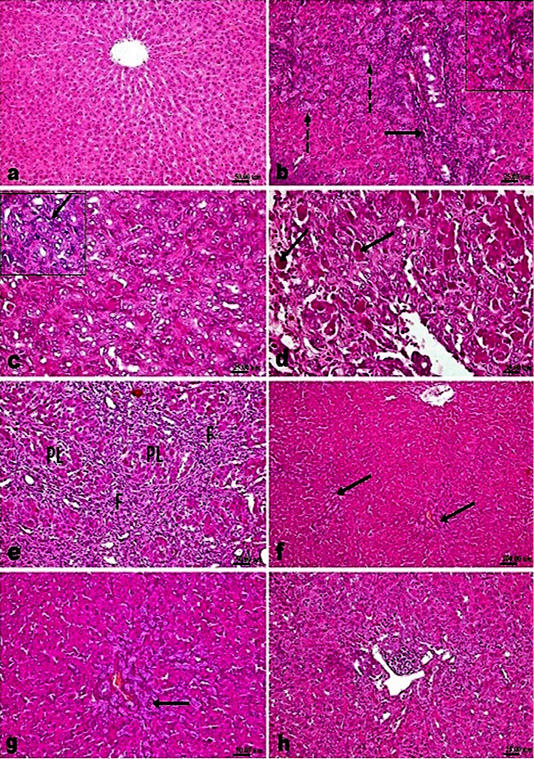
Figure 2: H and E stained sections; AM1241 interfered the progress of hepatic fibrosis associated with bile duct ligation (BDL); (a) Liver of control rat showing normal histological structure. (b-e). Liver of BDL rat showing; (b) marked proliferation of the bile duct epithelium with many newly formed bile ducteols (dashed arrows) and mononuclear inflammatory cells infiltration (arrow); (c) insinuation of the proliferated cholangiocytes among the parenchymal cells accompanying proliferated oval cells (arrow); (d) many apoptotic bodies and cells (arrow); (e) fibroplasia (F) with marked pseudolobulation (PL) of the hepatic parenchyma; (f and g) Liver of BDL rat that treated with Silymarin showing mild portal fibroplasia, moderate cholangiocytes’ proliferation and multiple newly formed bile ducteols (arrow); (h) Liver of BDL rat that treated AM1241 (3mg/kg bwt) showing marked decreased cholangiolar epithelial proliferation and few portal inflammatory cells infiltration.
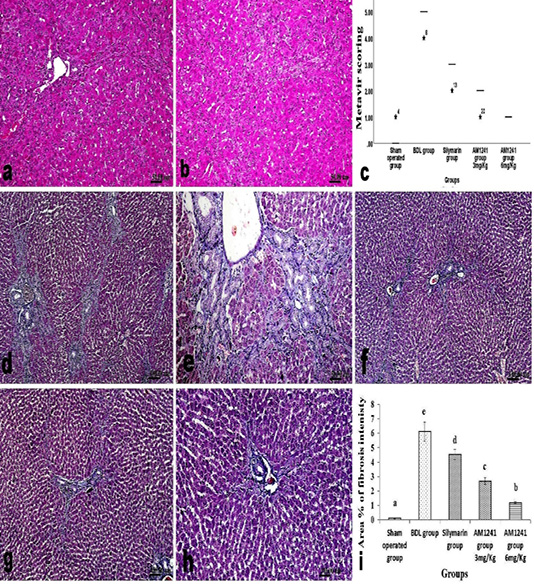
Figure 3: a and b; H and E stained liver sections of BDL rats that treated AM1241 (3 and 6mg/kg b wt, respectively) showing; (a) very mild portal fibroplasia without any evidence of peripheral extension; (b) explicit hepatic lobular regularity preservation; (c) Metavir scoring for fibrosis extension in control, BDL and different treated groups; (d- h) Masson’s Trichrome stained liver sections; (d and e) BDL rat showing accretion in hepatic fibrous components in portal triads and peripherally; (f) silymarin administration to BDL rats reduced fibrous tissue proliferation; (g and h) AM1241 administration to BDL rats significantly decreased fibrous tissue proliferation. (i) The intensity of fibrous proliferation in different groups evaluated by image analysis software (Image J, 1.46a, NIH, USA).
DISCUSSION
The current work aimed to assess the role of CB2 agonist on suppressing the progress of liver fibrosis induced by bile duct ligation (BDL) using synthetic analog (AM1241). In addition, evaluating the role provoked by CB2 receptors in modulating liver function, inflammation and oxidative stress.
Bile duct ligation is a common model for inducing obstructive cholestatic injury in rodents and considered as a classic experimental model for secondary biliary fibrosis. In a previous study, the maximum collagen deposition was obtained three weeks post-surgery, and the biochemical levels of liver enzymes and bilirubin reached maximum after three weeks (Tarcin et al., 2011). AM1241 has been classified as a protean agonist at the CB2 receptor (Yao et al., 2006). It was clearly indicated that; the gene expression of both CBI and CB2 receptors is elevated in liver fibrosis, however, CB1 receptors possess a pro-fibrotic effect, while CB2 receptors have anti-fibrotic effect (Pertwee, 2005; Yao et al., 2006).
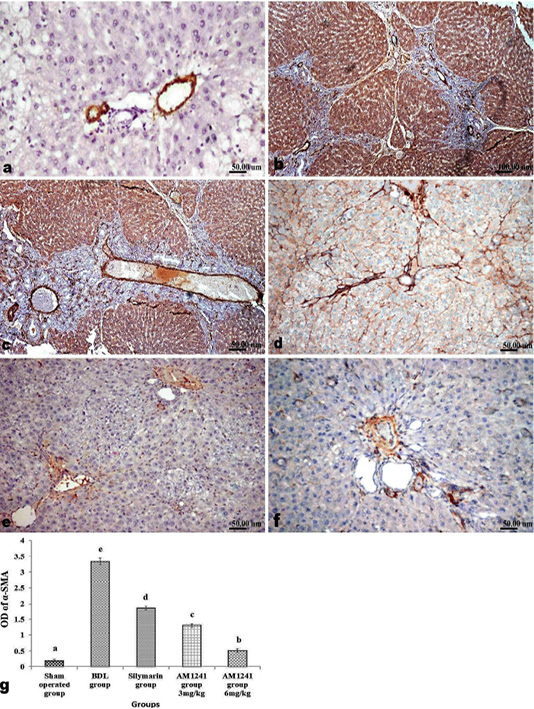
Figure 4: Immunohistochemical analysis of α-SMA; (a) control rat showing normal positive α-SMA expression around portal vein; (b and c) BDL rat showing increased expression of α-SMA in the portal areas and along the fibrous tissue proliferation; (d) decreased expression of α-SMA in silymarin treated rat; (e and f) marked limitation of α-SMA expression in AM1241 (3 and 6mg/kg b wt,) administrated rats; (g) Quantitative image analysis of α-SMA positive brown color in 5 microscopic fields (the area for each microscopic field is 18.8913Sqmm). Values are expressed as mean ± SE. Data were analyzed by using one way ANOVA followed by Tukey post-hoc test, a, b, c, d and e are significant at P < 0.05.
In the current work, bile duct ligation resulted in significant increase in liver function markers; AST, ALT, ALP, serum total and direct bilirubin which is in agreement with that mentioned by Tag et al. (2015). The later elevation in liver function markers could be attributed to the toxic effects of the accumulated bile salts as a result of bile duct ligation which caused hepatocellular necrosis and subsequently solubilized membrane phospholipids that led to release of the intracellular transaminases (AST and ALT) into blood circulation elevating their levels. The elevation in alkaline phosphatase (ALP) indicates a significant change in biliary flow associated with hepatobiliary disease which results from increased de novo synthesis in liver followed by its release into the circulation. Moreover, elevated serum levels of total and direct bilirubin are considered an index for cholestatic induced hepatic dysfunction as a result of bile duct ligation. Silymarin administration to BDL rats in the current experiment resulted in significant decrease in serum transaminases, ALP, total and direct bilirubin levels, those results came in accordance with the results of Bahmani et al. (2015). The later emended effect can be imputed to the capability of silymarin to decrease the entrance of toxic bile salts to hepatic cells by the membrane stabilization. The administration of AM1241 to BDL rats for two weeks brought about significant decrement in serum levels of the previous hepatic function markers, which is in accordance with Mallat et al. (2011), and it can be traced back to the heap to protective effect of CB2 receptors against various hepatic insults via the ability of CB2 receptors agonists to decrease; the expression of adhesion molecules, activation of endothelial cells, inflammatory response, and inflammatory cytokines, which all help the enhancement of liver tissue condition (Bátkai et al., 2007).
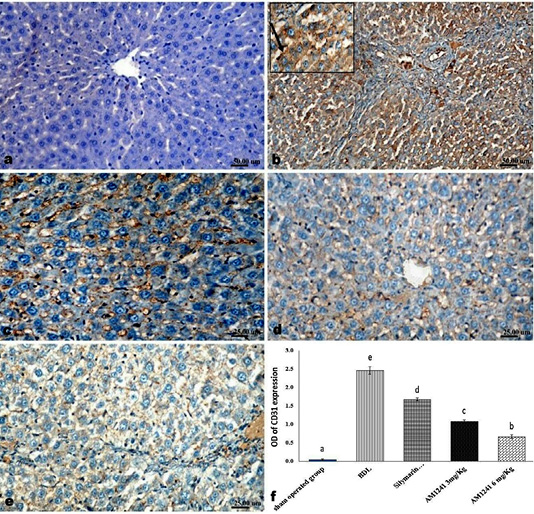
Figure 5: Immunohistochemical analysis of CD31; (a) control rat showing negative immune-expression of CD31; (b) BDL rat showing increased CD31 immune-reactive cells among the sinusoidal endothelial linings (c) decreased expression of CD31 in silymarin treated rat; (d and e) marked decreased expression of CD31 in AM1241 (3 and 6mg/kg b wt,) administrated rats; (f) Quantitative image analysis of CD31 expression in 5 microscopic fields (the area for each microscopic field is 18.8913Sqmm). Values are expressed as mean ± SE. Data were analyzed by using one way ANOVA followed by Tukey post-hoc test, a, b, c, d and e are significant at P < 0.05
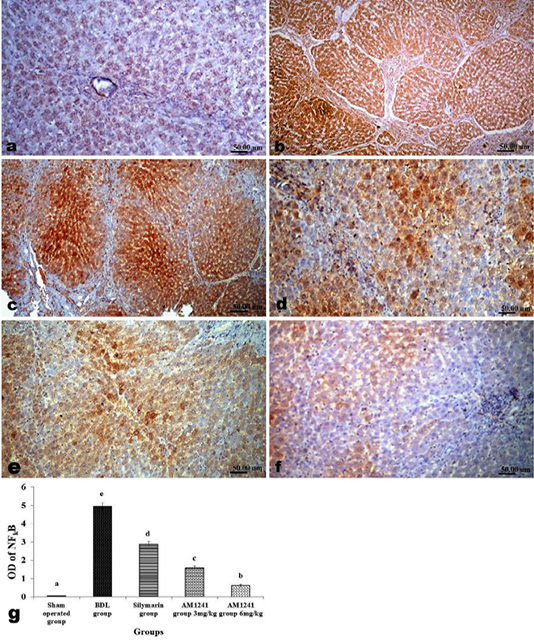
Figure 6: Immunohistochemical analysis of NF-κB; (a) control rat showing negative immune-expression of NF-κB; (b and c) BDL rat showing increased NF-κB among the hepatic parenchymal cells; (d) decreased expression of NF-κB in silymarin treated rat; (e and f) significant decreased expression of NF-κB in AM1241 (3 and 6mg/kg b wt,) administrated rats; (g) Quantitative image analysis of NF-κB expression in 5 microscopic fields (the area for each microscopic field is 18.8913Sqmm). Values are expressed as mean ± SE. Data were analyzed by using one way ANOVA followed by Tukey post-hoc test, a, b, c, d and e are significant at P < 0.05.
Oxidative stress was obvious in liver homogenate of BDL rats evidenced by significant increase in the level of the lipid peroxidation marker; MDA and a significant depletion in GSH content, which is in harmony with Mahmoud et al. (2014). Operated oxidative stress promoted with the generation of reactive oxygen species as well as when the other free radicals levels exceeds the antioxidant defense mechanisms. The accumulating ROS can cause oxidative damage to liver tissue and degrade the polyunsaturated lipids, forming MDA which released into the circulation in high levels. GSH is a part of the defense mechanism in biological system against lipid peroxidation, upon marked accumulation of free radicals in response to cellular injury as a result of bile duct ligation, GSH become depleted and its serum levels decrease (Yasui et al., 2005).
Silymarin administration to BDL rats significantly restored the antioxidant system and decreased lipid peroxidation manifested by maintained GSH and MDA levels. This restoration could be related to the protective antioxidant effect of silymarin, as it is possibly act as a scavenger for free radical, an inhibitor for lipid peroxidation and a preserver to GSH content (Kadir et al., 2018).
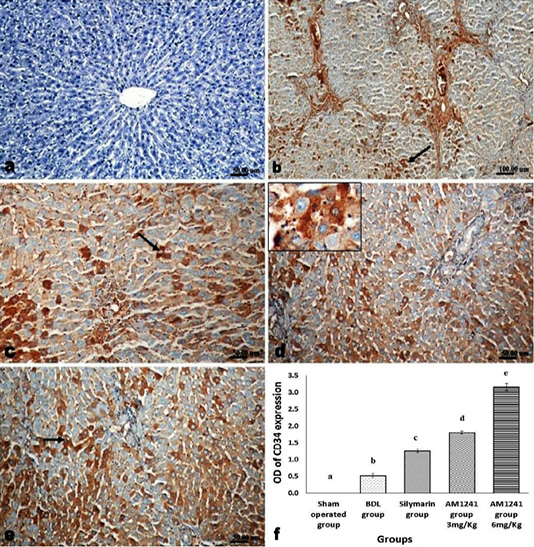
Figure 7: Immunohistochemical analysis of CD34; (a) control rat showing negative immune-expression of CD34; (b) BDL rat showing mild immunopositivity of CD34; (c) increased immune expression of CD34 in silymarin treated rat; (d and e) marked dose related increased CD34 immune-positive cells in AM1241 (3 and 6mg/kg b wt,) administrated rats; (f) Quantitative image analysis of CD34 expression in 5 microscopic fields (the area for each microscopic field is 18.8913Sqmm). Values are expressed as mean ± SE. Data were analyzed by using one way ANOVA followed by Tukey post-hoc test, a, b, c, d and e are significant at P < 0.05.
Administration of CB2 activator in the present work effectively retrieved the antioxidant defense system in liver which expressed by the marked amendment of MDA and GSH levels, that was coincided with a decrement in the inflammatory response observed histologically. The latter is in agreement with the results of previous studies which showed that; CB2 receptors activation by JWH133 and cannabidiol effectively decreased both hepatic ROS and the end-products of lipid peroxidation by attenuating the infiltration of inflammatory cells infiltration, particularly neutrophils and decreased the expression of neutrophil adhesion molecules in an animal model of I/R and alcohol-induced injury (Bátkai et al., 2007; Mahmoud et al., 2019).
In the current work, bile duct ligation resulted in significant increase in hepatic levels of IL-6 and TNF-α with a significant increase in immune-expression of NF-κB and TLR4 gene expression, which is in agreement with Oya et al. (2014). These findings can be returned to the overexpression of TLR4 which activates NF-κB that in turn works on enhancing the activation of HSCS by boosting the expression of pro-inflammatory cytokines; TNF-α and IL-6 followed by increased TGF-β1 and collagen expression, thus promoting liver fibrosis. The activation of TLR4 in bile duct ligation model occurs due to; BDL results in augmenting the chance of microbial translocation from intestine to liver which increases the levels of lipopolysaccharide (LPS) (Bai et al., 2013) that evoke LPS/TLR4 signaling pathway which involved in the transactivation of HSCs and causes liver fibrosis (Liu et al., 2015). Moreover; TNF-α and growth factors are strongly associated with free radicals generation and oxidative stress development leading to tissue fibrosis via inducing unbalanced synthesis of collagen, proteoglycan and hyaluronate assisting tissue remolding and fibrosis (Oya et al., 2014). The latter confirmed by our histopathological observation of increased tissue fibroplasia in BDL rats that asserted by increased Metavir score and percentage of fibrosis intensity. Silymarin administration resulted in significant decrement in TLR4 expression and levels of NF-κB, TNF-α and IL-6, which are in the same line with results of Al-Rasheed et al. (2014) who proved the inhibiting effect of silymarin on NF-κB which down-regulate IL-6 and TNF-α expression.
Our results showed that AM1241 treatment to BDL rats significantly decreased the activation of TLR4 and down-regulated NF-κB immune-expression as well as IL-6 and TNF-α, levels. The later cytokines work together on attenuating the activation of hepatic stellate cells (HSCs), and weaken inflammation. Lowering TLR4 expression can lead to decrease the levels of NF-κB, TNF-α, MyD88, IL-1 and IL-6 (Liu et al., 2015).
Generally, TLRs are the recognition receptors for the germline-encoded pattern which are fundamental as an initial step of the host defense mechanism against microorganisms in the innate immune system (Takeda and Yamamoto, 2010). It was suggested that; TLR4 has a direct effect of on fibrogenesis, and they may possess a role in hepatic damage and that way can modulate the disease progression. Furthermore, different cell types in liver express TLRs, including hepatocytes, Kupffer cells and hepatic stellate cells (HSCs). The substantially powerful influence of TLRs on inflammation could be related to their expression in liver diseases even in the early stages, proposed that TLRs act as an essential link between hepatic injury, inflammation and fibrosis (Pradere et al., 2010). As we mentioned earlier TLR4 have direct effect on NF-κB which is well known to play an important role in inflammatory response regulation; its activation can have an impact on different pro-inflammatory cytokines levels such as TNF-α, IL-1 and IL-6, as well as the levels of the pro-fibrogenic factors such as TGF-β1, thus can boost the proliferation and survival of activated HSCs.
In the current work, BDL rats showed significant decrease in the immunomodulatory cytokines; IL-10 which is in agreement with Osawa et al. (2013), which was significantly increased by silymarin and AM1241 administration to BDL rats. Correa et al., (2005) mentioned that JWH-133 (CB2 agonist) enhanced the release of IL-10 by LPS/IFN-y-activated macrophages through a pathway mediated by CB2 receptors activation. Modulating IL-10 receptor system can inhibit a range of macrophage functions that play a central role in hepatic inflammation and fibrosis.
Collectively, the activation of CB2 receptors by AM1241 in the current work resulted in deactivation of TLR4 gene expression via Myd88 signal transduction. Hence, when AM1241 affect MDY88 gene, it will subsidiary work on weaken the TLR4 expression which lower the NF-κB level that in turn will reduce the levels of different pro-inflammatory cytokines such as TNF-α, IL-1 and IL-6 as well as the levels of the pro-fibrogenic factors such as TGF-β1 and meanwhile increases the level of immunomodulatory cytokines (IL10). All of the above govern the inflammation and share to abort the ongoing fibrosis.
The observed histopathological alterations in BDL rats were similar to that mentioned by Marques et al. (2012) including, necrosis, portal hepatitis and marked cholangiolar proliferation accompanied with fibroplasia and pseudolobulation. Those histological alterations could be attributed to the unusual reflux of bilirubin and bile acids in liver, as well as the subsequent accumulation of toxic hydrophobic bile salts within hepatocytes resulting in progressive increase in hepatocytes necrosis with accompanying portal inflammatory response.
Silymarin administrated rats showed reduced hepatic fibrosis, that hepatoprotective effect may be related the diminishing effect of silymarin on Matrix metalloproteinases (MMPS), tissue inhibitors of metalloproteinases (TIMPS) and fibrogenic transcriptional factors as Kruppel-like factor 6 (KLF6) which all share in regulation of several pro-fibrogenic genes expressed in activated HSCs and led to interruption of collagen fiber formation (Chen et al., 2012).
Daily administration of AM1241 to BDL rats in the current work for 2 weeks induced marked improvement in the inflammatory reaction and collagen fiber deposition, a result which is in agreement with that of Mallat and Lotersztajn (2008). The later improvement could be attributed to; AM1241 could efficiently reduce TNF-α level thus decreasing both the inflammatory reaction and the release of ROS and other oxidative stress markers as well, thus interrupting the pathogenesis of cholestasis and hepatocyte death (both apoptotic and necrotic) (Bátkai et al., 2007). Moreover, it was reported that CB2 stimulation could inhibit hepatocytes apoptosis by its effect on the toll-like receptor complex CD14/TLR4/MD2 that intercede the production of pro-inflammatory cytokines including IL-1β and TNF-α (Gertsch, 2008). Furthermore, CB2 agonists can bind to and activate the key hepatic fibrosis cells (my ofibroblast and activated stellate cells), triggering potent antifibrogenic effects, namely; growth inhibition and apoptosis. Additionally, the antifibrotic role of endocannabinoid was found to depend on the signaling pathways initiated by the release of ROS following Δ9-tetrahydrocannabinol (THC) stimulation and instigating the apoptosis of human hepatic my ofibroblast (Julien et al., 2005).
The fibrogenic molecule; α-SMA showed increased expression in BDL rats, which is a major product of HSCs activation. However, activated hepatic stellate cells (HSCs) are considered the most important cells that are responsible for production of ECM and secreting collagen, which can lead to fibrosis (Oh et al., 2003). Silymarin administration to BDL rats, quietly limited the immune-expression of α-SMA which could be attributed to the direct anti-fibrgenic effects of silymarin on HSCS (Mahli et al., 2015). Whereas, daily administration of AM1241 to BDL rats had a significant reduction effect on hepatic expression of α-SMA, which was in agreement with (Mallat and Lotersztajn, 2008). The later effect could be a result of CB2 activation which decrease the presence of hepatic myofibroblasts and activated hepatic stellate cells that leads to decrease the expression of α-SMA and restoration of hepatic tissue (Mallat et al., 2013).
CD34 is a substantial marker for the hematopoietic stem/progenitor cells isolation, and it might be expressed in hepatic stem cell progeny. It is well-known that Oval/progenitor cells are capable of regenerating both hepatocytes and biliary cells following BDL. Additionally, it was reported that; CD34 is generally expressed in injured liver in large amount and in normal liver in low or nil amounts and is already expressed on three types of cells; hepatic stem cells, endothelial stem cells and bile duct epithelial cells (Lotowska et al., 2017).
Regarding, CD31; it is a cell surface protein that is expressed on endothelial cells and used as a signal for the extent of angiogenesis and vascularization. Sinusoidal endothelial cells expressed CD31 in a continuous manner from the portal space to the centrilobular vein, so, its expression and distribution used as EC marker (Pusztaszeri et al., 2006). In the current work, BDL rats showed increased immune-expression of CD31 which could be imputed to the angiogenic role of HSCs interposed by angiopoietin 1 that contributes to liver fibrosis development. Both of hepatic fibrosis and angiogenesis are mutually stimulatory, so that fibrosis progression requires angiogenesis, while angiogenesis requires angiopoietin 1 which is secreted from activated HSCs (Taura et al., 2008). Silymarin administrated rats in the present work, showed significant decrease in CD31 expression, which agree with Feher and Lengyel (2012) who mentioned the role of silymarin in regression of hepatic cancer due to its anti-angiogenic effect. However, AM1241 administration showed significant dose-related decreased expression of CD31 which point out a diminished angiogenesis. CB2 agonist; JWH‐015 ameliorated hepatic angiogenesis and fibrosis in BDL rats through down regulating CD31 and Vascular endothelial growth factor (VEGF) receptors VEGFR‐1 and VEGFR‐2 via inducing apoptosis of vascular ECs (Huang et al., 2012). In our work, BDL rats showed nil immune-expression of CD34 without any noticeable regenerating hepatocytes or oval cells. This came in harmony with the results of Sharma et al. (2013). Silymarin administrated rats showed moderate significant increase in CD34 positive cells, which could be imputed to the stimulatory effect of silymarin on liver tissue regeneration via increasing protein synthesis through increase the formation of ribosomes and DNA synthesis (Bahmani et al., 2015). While, AM1241 administration showed a dose related significant increase in CD34 positive cells, this effect could be related to AM1241 could activate Hepatic progenitor cells and promotes endogenous regeneration into functional hepatocyte-like cells, so, attempting to improve the potential of activated CD34 progenitor stem cell (Palazuelos et al., 2006).
CONCLUSION
To cut a long story short, our findings strengthen the role of CB2 receptors activation in suppressing ongoing liver fibrosis, promoting liver regeneration and down-regulating liver injury through featured mechanisms including; TLR4 dependent inhibition, suppression of some pro-inflammatory cytokines; IL-6 and TNF-α, boosting immunomodulatory cytokines; IL-10, weaken inflammation via down-regulating the levels of NF-κB, reducing the angiogenesis indicated by decreasing CD31 and stimulating oval/progenitor cells indicated by increased CD34 expression.
AUTHORS CONTRIBUTIONS
All authors contributed to the reagents/materials/analysis tools, wrote and revised the manuscript.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES






