Advances in Animal and Veterinary Sciences
Research Article
Short Term Iron Overload Injection Alters Reproduction Organ and Sperm Quality in Male Mice
Mas Rizky A.A Syamsunarno1,2,4, Rini Widyastuti3,4*, Herlambang Herlambang5, Alkaustariyah Lubis2, Mohammad Ghozali1,2, Ramdan Panigoro1,2
1Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jatinangor, Indonesia, 45363; 2Working Group of Genetic Medicine, Faculty of Medicine, Universitas Padjadjaran, Jatinangor, Indonesia, 45363; 3Laboratory of Animal Reproduction and Artificial Insemination, Department of Animal Production Animal Husbandry Faculty, Universitas Padjadjaran, Jatinangor, Indonesia, 45363; 4Central Laboratory, Universitas Padjadjaran, Jatinangor, Indonesia, 45363; 5Faculty of Medicine and Health Sciences, Universitas Jambi, Jambi, Indonesia, 36122.
Abstract | Background: Iron is an essential substrate in the body and needed in a small amount. In iron overload condition, such as patients who undergo routine blood transfusion for a long period, there is a risk of iron toxicity and affect fertility, signed by hypogonadism and reduction of sperm quality. However, the effect of short-term iron overload in the reproductive organ is not clearly understood. This study is conducted to determine the effect of short term iron administration to the male reproductive organ, testosterone, and epididymis sperm quality. Methods: Mice were injected with iron dextran intraperitoneally by the doses of 0, 0.05, 0.1, and 0.3 mg/day for 18 days. The reproductive organ, blood, and epididymis sperm were collected at the end of the treatment.
Results: Epididymis-body weight ratio was decreased and the seminal vesicle-body weight ratio was increased in iron dextran treatment group compare to control (p<0.05). The interstitial seminiferous tubules in testis showed the accumulation of debris cells and thickening of epithelial. and the seminiferous intratubular filled with fibrin deposit and disintegrated of spermatogenic cell. There was a significant increase in sperm concentration (p<0.05) and sperm motility rate in the iron dextran treated-group. Furthermore, the testosterone serum level was increased in treatment groups in a dose-dependent manner, although the difference was not statistically significant (p>0.05).
Conclusion: Short term iron overload alters the male mice reproduction showed by disruption in testis and increase of epididymis sperm concentration and motility (p<.0.05)
Keywords | Iron overload, Sperm quality, Testis, Testosterone, Histopathology
Received | September 05, 2020; Accepted | September 16, 2020; Published | December 05, 2020
*Correspondence | Rini Widyastuti, Pathology Department, Faculty of Veterinary medicine, Zagazig University, Egypt; Email: [email protected]
Citation | Syamsunarno MRAA, Widyastuti R, Herlambang H, Lubis A, Ghozali M, Panigoro R (2021). Short Term Iron Overload Injection Alters Reproduction Organ and Sperm Quality in Male Mice. Adv. Anim. Vet. Sci. 9(1): 35-41.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.1.35.41
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Widyastuti et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Iron is a crucial micronutrient which plays an important role in numerous biological function including oxygen and electron transport, redox reactions, cell division, nucleotide synthesis, and myelination (Kühn, 2015). Iron consists of heme and iron-sulfur group and bound to ferritin or transferrin, as it is free form toxic as it can catalyze Fenton reactions and form hydroxyl radical that can injure tissues. Iron homeostatic is regulated through the hepcidin-ferroportin axis. The body iron content and distribution are regulated by four cells, namely duodenal enterocytes (affecting dietary iron absorption), erythroid precursors (affecting iron utilization), reticuloendothelial macrophages (affecting iron storage and recycling), and hepatocytes (affecting iron storage and endocrine regulation) (Neves et al., 2017). Imbalance of the homeostasis causes iron overload syndromes which are primary (hereditary hemochromatosis) or secondary (transfusional and dietary). Excess iron will be deposited throughout the body and most notably heart, liver, and endocrine glands. These contributed to the manifestation such as diabetes mellitus, insulin resistance (IR), and nonalcoholic fatty liver disease (Neves et al., 2017).
The exact mechanism whether gonadal dysfunction due to primary iron deposits in the gonads or secondary to a hypogonadotropic state in patients with iron overload is still controversial. Laboratory evaluation must be conducted including endocrinological examination, evaluation of serum basal level of follicle-stimulating hormone (FSH), luteinizing hormone (LH), free testosterone, and GnRH as well as a standard semen analysis. A previous report stated that normal sperm quality is impaired in men with iron overloading conditions (Safarinejad, 2008). In women, the toxic effect reduces gonadotropin secretion in the anterior pituitary gland, and thereby hypogonadotropic hypogonadism and subfertility occurred (Noetzli et al., 2012).
Beta-thalassemia women who undergoing routine blood transfusion has been reported success in conceiving, whereas paternity in Beta-thalassemia men did not occur. However, successful spermatogenesis ensues in oligoasthenospermia. This indicates male Beta-thalassemia patients respond less well than their female counterparts. Iron-chelating therapy has an important role in long-term survival and quality of life for patients with transfusion-dependent beta-thalassemia. Nevertheless, the recent micromanipulation technique such as ICSI has improved the chance of conception even in oligoasthenospermic patients (Bajoria and Chatterjee, 2011). Therefore, the preservation of the reproductive function of male patients with iron overloading conditions as in beta-thalassemia has become an important issue (Chen et al., 2018).
Testis rich in mitochondria and iron is required in metabolism and respiration (Kreb’s cycle and electron transport). Subsequently, iron is delivered to germinal cells by testicular transferrin and bind transferrin receptor in early spermatocyte. Other proteins such as frataxin and mitoferrin were also expressed in testis mitochondria (Neves et al., 2017; Leichtmann-Bardoogo et al., 2012). The accumulation and deposit of iron in testis lead to male infertility (Maccarinelli et al., 2017). However, the effect of short term iron overload in the reproductive organ is not clearly understood. The purpose of this study was to determine the effect of short term iron administration to the male reproductive organ, testosterone, and epididymal sperm quality.
Materials and Methods
Animal Treatment
The study was approved by the Ethical Committee on Research of the Universitas Padjadjaran, Bandung, Indonesia (No:43/UN6.C1.3.2/KEPK/PN/2017). Eight to ten weeks old male white mouse (Mus musculus) with bodyweight approximately 25-30 grams were purchased from PT Bio Farma, Bandung, West Java, Indonesia. The animals were acclimatized for seven days to adapt with new environment and maintained in cages at room temperature and a 12-hour light/dark cycle with adequate air circulation. All mice were provided with food and water ad libitum. The animals were divided into four groups, each mice in the treatment groups were injected intraperitoneally with iron dextran (Hemadex, Sanbe, Indonesia) with a dose of 0.05, 0.1, and 0.3 mg/day in 200 μl of NaCl 0.9%. The dose was based on the previous study and calculated to finding Animal Equivalent Dose (AED) by multiplying human dose (mg/kg) with the Km ratio (Nair and Jacob, 2016, Panigoro et al., 2018).
Samples Collection
Mice overnight fasted and the body weight was measured at the end of the treatment day. Mice were euthanized by ketamine/xylazine before sample collection. Testis, epididymis, and seminal vesicle were isolated, removed from adherent tissues and blood, then the weight was recorded. The reproductive organ weight was represented as a percentage of organ weight (g)/body weight (g). Blood was collected from an abdominal vein for further analysis.
Sperm Concentration
Sperm were collected from cauda epididymis then diluted in 1 mL of NaCl. Ten microliters of mixtures were transferred into a hemocytometer and allowed to settle. The cells that quiescent inside the Neubauer chamber were counted to calculate sperm concentration followed by observation using an upright microscope with 200x magnification (Olympus CX 21, Japan) (World Health, 2010).
Sperm Motility
Sperm motility was observed under an upright microscope with 100x magnification (Olympus CX21, Japan). A drop of diluted sperm was placed in a glass slide then covered with a cover glass. The motile and nonmotile sperm were counted with a total of 200 cells. The percentage of motility was calculated as motile sperm/200 cells x 100%.
Histological Analysis
The testis was fixed in 10% formaldehyde, embedded in paraffin, continued with sliced to 5 µm then stained using hematoxylin and eosin (HE). The sections were observed for the histological analysis with 200x magnification (Olympus IX71, Japan).
Testosterone Serum Level Assay
Blood was centrifuged at 3000 rpm to separate the serum for testosterone quantification. The quantitative determination of the hormone was conducted by using Enzyme Immune Assay Method (ELISA). The Mouse testosterone ELISA kit (EM1850) was obtained from Fine Test (Hubei, China). The concentration of testosterone was assayed according to the procedures outlined in the respective manufacturer’s protocol.
Statistical Analysis
The statistic was analyzed with IBM SPSS Statistics 20 using analysis of variance (ANOVA) and Tukey post hoc to evaluate the differences between groups. P-value <0.05 was considered as statistically significant.
RESULTS
The tests, epididymis, and seminal vesicles are all androgen-dependent organs, relying on testosterone for their growth and function. The changes of testis, epididymis, and seminal vesicles weight-body weight ratio (TW/BW, EW/BW, and SVW/BW respectively) induced by iron dextran injection may reflect the changes of bioavailability and production androgen. The reproductive organ-body weight ratio in the control and iron dextran treatment group is presented in Figure 1.
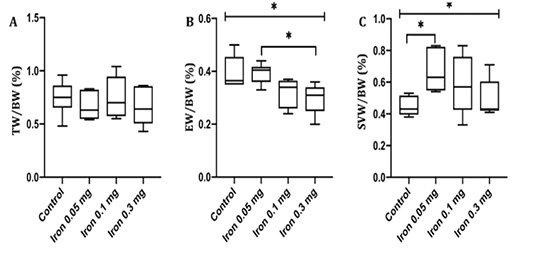
Figure 1: Testis weight-body weight ratio (A) epididymis weight-body weight ratio (B) and seminal vesicles weight-body weight ratio (C) in control and treatment group measured after 18 days of iron dextran. TW= Testis weight; BW= Body weight; EW= Epididymis weight; SVW= Seminal vesicles weight; *= p<0.05
TW/BW ratio was not different between control and all group of treatment. A prominent decrease in EW/BW was observed in the group injected with 0.1 and 0.3 mg/day of iron dextran compared to control. In contrary, SVW/BW ratio was increased significantly in dose 0.05 and 0.3 mg/day of iron dextran compared to control (p< 0.05).
We examined the histology of testis by HE staining (Figure 2). In general, there was severe deterioration of histology of testis in the treatment group in a dose-dependent manner. The histological analysis showed that Sertoli cells were distributed in the tubules surrounded by the well-integrated smooth muscle. Leydig cells were spread simultaneously in the interstitial. In group of iron dextran dose 0.1 and 0.3 mg/day, the interstitial seminiferous tubules showed accumulation of debris cells and thickening of epithelial. Seminiferous intratubular filled with fibrin deposit disintegrated of spermatogenic cell, and disintegrated of epithelial tubule wall.
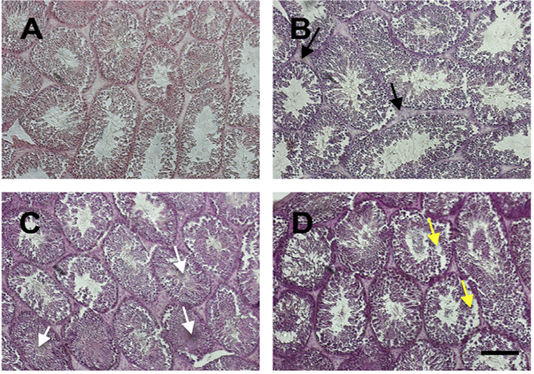
Figure 2: The histology of testis stained by HE staining in control (A) and treatment groups (B-D; Iron dextran 0.05, 0.1 and 0.3 mg/day respectively). Treatment groups showed interstitial cell hyperplasia (black arrow), fibrin coalescent in the center of tubules (white arrow) and some of the spermatogonium and spermatocyte cells were detached from the basalis membrane (yellow arrow). Scale bar=100 μm.
Sperm concentration and motility were examined to confirm whether iron dextran influenced sperm quality. Despite the deterioration in testis histology, there was a significant increase in sperm concentration (p<0.05) and sperm motility rate in iron dextran treated-groups (Figure 3A and B). The sperm concentration was slightly decreased in iron dextran dose 0.3 mg/day but still higher compared to the control group (Figure 3A). The sperm motility tended to increase in the treatment group although it was not statistically significant (Figure 3B).
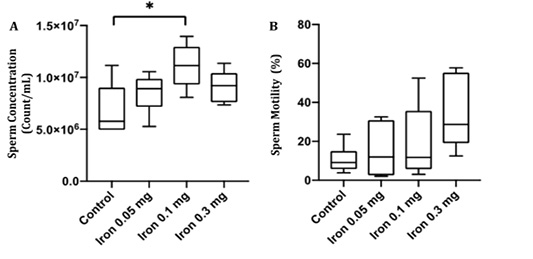
Figure 3: The epididymal sperm concentration (A) and motility (B) in the control and treatment group after 18 days of iron dextran injection.*=p<0.05.
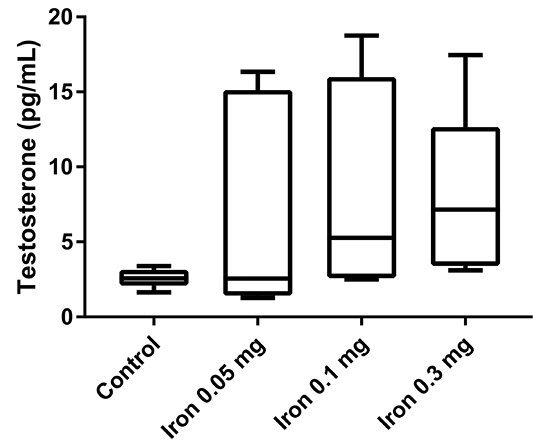
Finally, the testosterone serum level was measured to confirm whether iron dextran can affect testosterone serum concentration (Figure 4). After 18 days of iron dextran injection, testosterone serum level was increased in treatment groups in a dose-dependent manner compared to control, although the difference was not statistically significant.
DISCUSSION
Iron is an essential mineral that plays a role in the biological process in various organs, including testis. However, the excess of iron can cause toxicity and damage the organ tissues (Anderson and Vulpe, 2009). In this study, we showed the effect of iron overload in several doses to the quality of epididymal sperm. There were no differences in testis weight among groups. In the previous study, Wistar rats that were treated with iron dextran 250 to 1,000 mg/kg body weight for 20 hours showed no differences among control and treatment groups (Lucesoli et al., 1999; Anderson and Vulpe, 2009). Iron was accumulated in the interstitium, interstitial macrophages, fibroblasts, and Leydig cells. There was a disruption in microstructure, particularly in high dose iron dextran injection groups, signed by thickening of the basal membrane, atrophied spermatic tubules, out of sperm cells in the tubular lumen, and moderate fibrosis in the interstitium (Lucesoli et al., 1999). In our study, the histology of testis was also disrupted. There was hyperplasia of interstitial cells of the treatment group compared to control. Fibrin was seen and coalescent with sperm in the middle of the lumen. The detached spermatogonium and spermatocyte are also presented. The hyperplasia indicates that the active proliferation of cells may be caused by an increase in hormonal activity. However, the cell damage may also be caused by oxidative stress activity of iron accumulation in the cells.
Our study showed epididymis weight was decreased while seminal vesicle weight became bigger in iron dextran treatment groups. Epididymis has function in maturating and storing sperm post testicular and seminal vesicle plays a role to produce semen for supporting sperm quality (Jones, 1999; Gonzales, 2001). The decrease in epididymis weight may be caused by the direct effect of iron deposits in gonad organ that induce oxidative stress and cell death. Enlargement of seminal vesicle weight indicated there was inflammation due to iron toxicity, hypertrophy in seminal vesicles, and or accumulation of semen that can not be distributed out from the gland. It is also suggested that seminal vesicle is not affected or in minimum affected by iron toxicity.
Iron overload condition, as seen in beta-thalassemia patients with chronic blood transfusion as therapy, has been correlated with disruption reproductive abilities in both males and females (Safarinejad, 2008; Chen et al., 2018; Singer et al., 2011; Chang et al., 2011). Our study showed that sperm concentration increase after iron overload injection. This is contrary to the previous study that reported most of male beta-thalassemia patients with chronic blood transfusion have abnormal sperm quality, including oligospermia and due to hypogonadotropic hypogonadism (Safarinejad, 2008; Chen et al., 2018). Iron is necessary for the spermatogenesis for the synthesis of material genetic and growth of germ cells. For example, Sertoli cells transport iron in the form of ferritin to spermatogonia and spermatocytes I. Sertoli cells also distribute iron to new spermatocytes at the spermatid stage (Leichtmann-Bardoogo et al., 2012). The capacity of iron transport from circulation to germ cells is limited by the blood-testis barrier. Regarding the different findings between previous studies and our study, it must be explained that iron has a minimum dose to cause enhancement or defect in sperm production.
In the present study, the mice sperm motility increase in dose-dependent although not significant. The highest dose of iron injection (0.3 mg/day) showed a three times increase in motility percentage compared to control. It has been shown that low iron concentration is correlated with azoospermia (low or no sperm) and asthenozoospermia (decrease of sperm motility) (Skandhan et al., 2012). The study of Kasperczyk et al. (2016) clearly described the sperm quality between males with low and high-iron concentration in seminal plasma. The iron level in seminal plasma does not affect total sperms count or percentage of motile sperm cells. However, progressive sperm motility is significantly higher in subject seminal plasma with low iron concentration compared to high iron concentration. The level of superoxide dismutase (SOD), an intracellular antioxidant, is also higher in subject seminal plasma with low iron concentration compared to high iron concentration (Kasperczyk et al., 2016). In vitro study using bull sperm incubated with iron in different concentrations indicated that iron in high dose has a toxic effect and iron in low concentration promotes spermatozoa activity (Tvrdá et al., 2015). In line with our study, Soliman et al. study found there were an increased of sperm quality parameters followed by an increased in concentrations of serum sex-related hormone in thalassemia male patient after seven days of blood transfusion (Soliman et al., 2012). Iron in free form has high reactivity with oxygen by the Haber–Weiss and/or Fenton reaction resulting in overproduction of reactive oxygen species (ROS). Consequently, high ROS disrupts axoneme protein phosphorylation and can cause sperm immobilization. The high level of ROS can also increase DNA damage (Pereira et al., 2017). To overcome this condition, SOD activity will increase to overcome a high level of ROS. SOD activity in short term iron overload will increase in tissue, such as in liver tissue (Aziza et al., 2014). However, in chronic iron overload, the capability of SOD will decrease as seen in subject seminal plasma with high iron concentration (Kasperczyk et al., 2016). Regarding the different results of sperm motility, it might be explained by the different duration of iron overload exposure. In a short time duration of iron overload, the antioxidant activity, such as SOD, might be an increase in seminal plasma to overcome the ROS produced by high iron levels.
Some studies indicated the level of testosterone correlates with iron metabolism (Shin et al., 2016; Carrero et al., 2012; Ferrucci et al., 2006). Studies of men with low testosterone levels have lower hemoglobin and hematocrit levels, while men with anemia of unknown etiology are more likely to have low testosterone levels compared to controls (Roy et al., 2017; Shin et al., 2016; Guo et al., 2014). Testosterone replacement therapy ameliorates anemia conditions both in aging men and mice (Bachman et al., 2014; Guo et al., 2014). It is already known that testosterone regulates iron metabolism by inhibiting hepcidin, a 25-amino acid peptide that responsible to iron absorption in the intestine and iron distribution to tissues (Nemeth and Ganz, 2009; Latour et al., 2014). The exogenous testosterone reduces hepcidin concentration and administration of finasteride, a 5α-reductase reductase, which converts testosterone to dihydrotestosterone, resulted in no change in hepcidin concentration. The proposed mechanism is testosterone directly increases the affinity of the androgen receptor for Smad1/Smad4 and cause reduction of androgen receptor to activate the hepcidin promoter. Testosterone can also activate the epidermal growth factor receptor that can also inhibit hepcidin (Guo et al., 2014; Bachman et al., 2014). So, the measurement of male reproductive hormones, including FSH, LH, and testosterone is imperative to be investigated for further study.
The mechanism of how testosterone regulates iron is depicted in many studies. However, the mechanism of how the iron level regulates testosterone is not well understood. The previous studies limited to chronic iron accumulation studies in patients with hereditary hemochromatosis, chronic blood transfusion, and people who eat food with iron fortification (Charbonnel et al., 1981; Kim et al., 2013; Schümann, 2001). Hypogonadism is a common complication of chronic iron overload together with heart failure and diabetes. Hypogonadism in males with chronic iron overload is caused by iron accumulation in the gonadotropic cells in the anterior of the pituitary gland. Thus, this impairment is also termed as hypogonadal hypogonadism or secondary hypogonadism (Tvrda et al., 2015; El Osta et al., 2017).
Despite the understanding of iron overload can induced hypogonadism, our result showed testosterone serum level tended to increase in mice that were injected with iron dextran. This finding might be due to the difference in the duration of iron supplementation (short term vs chronic iron supplementation). Macchi et al. (2017) reported that mice fed with an iron-enriched diet showed severe hypogonadism with a significant reduction of body weight gain, as well as serum levels of testosterone and LH, body fat, and plasma leptin (Macchi et al., 2017). The subtle increase of testosterone in our studies might also indicate that the body can adapt in the early phase of excessive iron conditions and chronic iron accumulation in the pituitary and reproductive organ can cause hypogonadism conditions. Further study for measuring the level of hepcidin and other iron metabolism-related protein is necessary to see how the male reproduction system adapts to excessive iron in the early phase.
ACKNOWLEDGEMENT
We would like to thank Neni Anggraeni and Asep Saepuloh for great assistance during the experiment. This research is supported by Academic Leadership Grant Universitas Padjadjaran on behalf of Professor Ramdan Panigoro.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest
Authors contribution
MRAAS, RW and AL design and performing experiment, data analyses. HH and MG and RP interpretation the histological data, literature and data analysis. All of the author preparing and writing the manuscript.
REFERENCES