Advances in Animal and Veterinary Sciences
Research Article
Indentification of Caspase 3 Intensity in Kacang Goat Oocyte Post Vitrification using Confocal Laser Scanning Microscopy
Widjiati Widjiati1, Zakiyatul Faizah2, Ninik Darsini2, Viski Fitri Hendrawan3, Helly Nurul Karima4, Choirunil Chotimah4, Sutiman Bambang Sumitro5, Epy Muhammad Luqman6*
1Post Graduate School of Universitas Airlangga Surabaya, Indonesia; 2Department of Biomedical Science Faculty of Medicine Universitas Airlangga Surabaya, Indonesia; 3Department of Reproduction Faculty of Veterinary Medicine Universitas Brawijaya Malang, Indonesia; 4Bio-Science Central Laboratory Universitas Brawijaya Malang; 5Department of Biology Faculty of Science Universitas Brawijaya, Malang; 6Department of Veterinary Anatomy Faculty of Veterinary Medicine Universitas Airlangga Surabaya, Indonesia.
Abstract | The research was aimed to find out caspase 3 intensity in Kacang goat oocyte post vitrification. The high caspase intensity will affect the viability of post thawing oocytes so that it will affect the oocyte quality and fertilization rate. The intensity of caspase 3 expression occurs because of the bond between caspase 3 with the Flou 3 probe so it will emit a fluorescent color, which is captured by the CLSM microscope device. The benefits of this research were to measure the intensity of caspase 3 quantitatively so as to determine the oocyte quality post thawing and determine the quality of frozen oocytes as a source of gametes for fertilization purposes. There were 2 groups in the research; Kacang goat fresh oocyte and kacang goat oocyte that was vitrified (each group consisted of 5 replications). The procedure of this study including oocyte collection, oocyte vitrification, frozen oocyte warming and Caspase 3 intensity examination were labeled with anti rabbit Fluorescein Isothiocyanate (FITC) secondary antibodies and measurement with Confocal Laser Scanning Microscopy (CLSM). Caspase 3 intensity of both groups was observed using CLSM. The results showed that caspase 3 intensity in the oocyte group which had been frozen by vitrification was higher significant than fresh oocytes (P < 0.05). The research concluded that caspase 3 intensity of frozen oocyte post vitrification was higher than that of fresh oocyte.
Keywords | Oocyte, Kacang goat, Vitrification, Caspase 3 intensity, Confocal Laser Scanning Microscopy
Received | July 14, 2020; Accepted | July 22, 2020; Published | November 15, 2020
*Correspondence | Epy Muhammad Luqman, Department of Veterinary Anatomy Faculty of Veterinary Medicine Universitas Airlangga Surabaya, Indonesia; Email: [email protected]
Citation | Widjiati W, Faizah Z, Darsini N, Hendrawan VF, Karima HN, Chotimah C, Sumitro SB, Luqman EM (2020). Indentification of caspase 3 intensity in kacang goat oocyte post vitrification using confocal laser scanning microscopy. Adv. Anim. Vet. Sci. 8(12): 1362-1366.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.12.1362.1366
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Luqman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Method to store Kacang goat oocyte as oocyte bank is by freezing. There are many freezing methods such as slow frezzing, rapid frezzing dan ultra rapid frezzing. At the moment a method frequently used to freeze oocyte is vitrification. Vitrification uses high concentration cryoprotectant in order to prevent from ice crystals formation so that it does not ruin oocyte membrane.
When frozen oocyte is used as source of gamete cell for in vitro fertilization, frozen oocyte is warmed. During warming, change of temperature, viscosity, osmotic pressure and intracellular liquid volume takes place so that oocyte often ruins (Jang et al., 2017). Temperature change during warming induces temperature stress or heat shock that results in Reactive Oxygene Species (ROS) therefore, it disturbs intracellular and extracellular oocyte. Also, temperature change induces to release free radicals so oocyte will make compensation by increasing HSP 70. If endogenous anti-oxidant is not able to ward off free radical release due to ruined protein structure, nucleit acid, lipid in membrane, and other organelles such as mitochondria, cytoskleton, spindle yarn so it ruins oocyte function (Chen and Yang 2009; Redza-Dutordoir and Averiil-Bates, 2016).
Increase of free radicals due to temperature change during warming induces over-expression of HSP 70 which is unable to intrinsicly keep oocyte from apoptosis. Therefore, mithocondria will release Apoptosis Protease Factor I (APAF –I) which activates to release cytochrome C, caspase 9 dan caspase 3 as executor of apoptosis to exist in oocyte post warming (Ravagnan et al., 2001).
Caspase 3 is one of caspases that has important role for apoptosis to take place intrinsicly or extrinsicly. Caspase 3 in oocyte post warming will induce change of molecular mechanism in oocyte and regulate oocyte maturation especially cytokine that has role in maturation process such as Maturation Promoting Factor (MPF) (Tomoya and Yamashita, 2002). Change of molecular mechanism due to caspase 3 increase in oocyte will affect oocyte viability, so it will decrease fertilization rate and embryo growth. The other side post warming oocytes have lower viability, fertilization an embryonic growth (De Munck et al., 2013).
Intensity of caspase 3 in oocyte post vitrification is able to be measured using Confocal Laser Scanning Microscopy (CLSM) method with Fluorescein Isothiocyanate (FITC) indicator. Using CLSM method, oocyte is sliced using laser to quantitatively measure caspase 3 in oocyte. Luminescence of colors released from FITC exposure will be captured and expressed in graphs so that intensity is able to be scored qualitatively.
The novelty of measuring caspase 3 intensity was an indicator of the quality of post thawing oocyte quality before fertilization and preimplantation. So far, measuring apoptosis as an indicator of oocyte quality was only quantitatively with immunocytochemistry (ICC), with CLSM being able to measure oocyte quality based on apoptotic indicators using caspase 3 qualitatively to the nucleus and cytoplasm.
MATERIALS AND METHODS
This research received ethical clearance number: 1.KE.061.04.2019 released by Animal Care and Use Committee, Universitas Airlangga, Faculty of Veterinary Medicine. The research was carried out to observe caspase 3 intensity in kacang goat oocyte post vitrification using examination of Confocal Laser Scanning Microscopy (CLSM). The research was designed into 2 groups, that is, Treatment I group (T1): caspase 3 intensity of frozen oocyte was observed and Treatment group II (T2): caspase 3 intensity of fresh oocyte was observed with CLSM and each group consisted of 5 replications (2 oocytes in one straw). Research stages consisted of oocyte collection, oocyte vitrification, frozen oocyte warming, caspase 3 intensity Examination using CLSM.
Oocyte Collection
Kacang goat’s ovaries were collected from local slaughterhouse with their reproductive history being unknown. Oocytes used in this study have grade A with specifications had cumulus–oocytes complex (COC) consisted of oocytes that were surrounded by five or more layers of compact cumulus cells (Bakri et al., 2016). Oocyte was cleaned and washed with physiological NaCl 0.9% which was supplemented with gentamycin sulfate 50 µl and warmed at waterbath with temperature of 30-35oC. Oocyte was collected using aspiration of follicles with diameter surface of 8-12 mm using disposable syringe 10 ml with 18 G needle. Collected oocyte was cleanly washed with minimum essential medium (MEM medium) (Wani et al., 2000; Islam et al., 2007).
Oocyte Vitrification
Oocyte was put into equilibration medium PBS + 20% serum + 0.5M sucrose + 15% ethylene glycol + 15% PROH (V2) for 18-20 minutes. After oocyte inflated perfectly, it was moved to M2 medium- (Sigma, Saint- Quentin-Fallavier, France) (V3) for 30 seconds. Next, oocyte was put at tip of hemistraw, vaporized on liquid N2 and put into liquid N2 (Faizah et al., 2016).
Oocyte Warming
Frozen oocyte was thawed by being exposed to warming medium step by step: PBS + 20% serum + 0.5M sucrose (V4) for 1 minutes, PBS + 20% serum + 0,25M sucrose (V5) for 2 minutes and PBS + 20% serum + 0.1 M sucrose (V6) for 3 minutes (Arnault et al., 2008; Faizah et al., 2016).
Caspase 3 Intensity Examination using CLSM
Frozen oocyte which was thawed was fixed then was washed with PBS 3 times for 5 minutes. Next, PBS was discarded and changed with 0,1 Triton X-100 in PBS and incubated at room temperatue for 30 minutes. After that, Triton x – 100 solution was discarded and washed with PBS as much as 3 times for 5 minutes. Then it was incubated with blocking buffer 3% bovine serum albumin (BSA) in PBS for 30 minutes, room temperature. Blocking buffer solution was discarded and Ab primary caspase 3 and Ab goat were supplemeted all night long with temperature 4 0C. It was washed with PBS 3 times for 5 minutes. It was incubated with Ab secundary Anti Rabbit FITC and Anti mouse Tetramethyl Rhodamine Iso-Thiocyanate (TRITC) for an hour, room temperature. It was washed with PBS 3 times for 5 minutes. Finally, it was observed using CLSM (Arnault et al., 2008).
Caspase 3 intensity in oocyte was able to be measured quantitatively using CLSM. Work mechanism of CLSM: oocyte which was exposed by Fluo 3 solution as indicator would reflect fluoroscent light which was captured and measured quantitatively. At CLSM method, oocyte supplemented with probe of Fluo-3 showed color luminescence with different wavelength which was then captured by filter presented into dye and put into chennel. Oocytes were labeled with anti rabbit FITC secondary antibodies and then the oocytes were observed with a Confocal Laser Scanning Microscope (CLSM) with a wavelength of 488 nm and analyzed with Olympus Fluoview software (Liu et al., 2015).
Statistical Analysis
Oocytes recovered from five Kacang goat were randomly assigned to the control and treated groups (2 oocytes in one straw). The data presented as means Standard Error of the Mean (S.E.M) and were compared using the Student’s t-test. They were considered to be significantly different if P < 0.05.
RESULT AND DISCUSSION
Data on caspase 3 intensity from examination using CLSM method which used Fluo 3 probe at 2 groups of Kacang goat oocytes. After being analyzed, it is presented at Table 1.
Table 1: Mean and standart deviation of caspase 3 intensity of Kacang goat frozen oocyte post vitrification and fresh oocyte without freezing
| Group | Mean ±SD |
| T1 (Oocyte frozen by vitrification method |
816.40 ± 27.34a |
| T2 (Fresh oocyte) |
669.24 ± 17.20b |
Different supercrips in the same column have significant difference P<0.05
Oocytes that were frozen will occur coldshock, a drastic change in temperature when frozen and when thawing will affect the micro molecules present in oocytes compared to fresh oocytes that were not frozen. At the time of coldshock, there would be an increase in HSP 70 to compensate for damage, if it did not succeed in compensating for damage, it will affect the increase in proinflammatory cytokines (Peraçoli et al., 2013). Protein HSP-70 was anti-apoptosis protein. HSP 70 would increase during oocyte freezing process due to temperature shock to give protection effect on oocyte. If the increase of HSP 70 was unable to give protection, caspase-3-like protease would activate apoptosis signaling route due to release of cytochrome c from mitochondria to cytosol and activation of caspase-3-like protease would induce apoptosis. Caspases were involved in regulating maturation process and related to cell degeneration (Redza-Dutordoir and Averiil-Bates, 2016; Arnault et al., 2008). From the results of caspase 3 research involved in the regulation of meiosis rhythm (Figure 1). Caspase-9 and caspase-3 on the meiotic spindle of oocyte metaphase II suggest that they may be involved in the regulation of meiosis, by participating in the development of normal meiosis or the early stages until death. The proof of the role on caspases in vivo in healthy follicles and atretic follicles to be able to determine the caspases involved in oocyte orientation towards ovulation or apoptosis (Arnault et al., 2008).
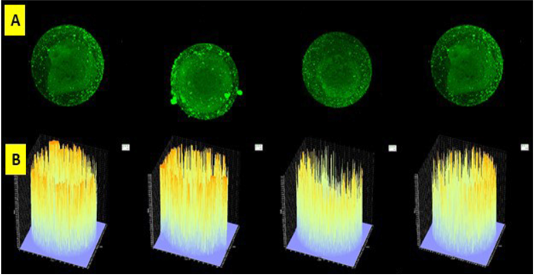
Figure 1: Slice of oocytes with a thickness of 5 µ (A) and read with Olympus Fluoview software version 4.2a. Caspase 3 intensity of frozen oocyte post vitrification group emitted by fluorescent luminescence in caspase 3’s oocyte that binds to secondary antibodies labeled FITC. The higher the intensity of caspase 3 in the oocyte indicated the higher the intensity and profile numbers on the graph were showed by the line density in yellow. The expression and intensity of caspase 3 of frozen oocyte post vitrification group were very high (B).
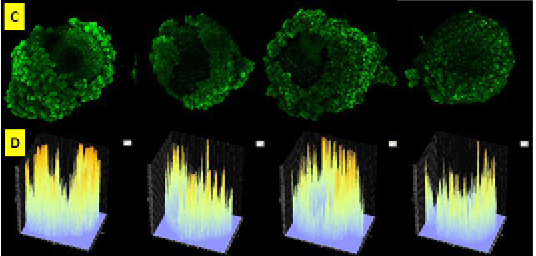
Figure 2: Oocyte slices before checked with Olympus Fluoview software version 4.2a emitted by green fluorescence (C). Graphic image of the intensity of caspase 3 fresh oocyte group emitted by fluorescent luminescence in caspase 3 oocyte that binds to secondary antibodies labeled FITC. The expression and intensity of caspase 3 of fresh oocyte group were lower than frozen oocyte post vitrification group (D).
Cryopreservation technique enabled long storage of various types of biological substances without significant damage of quality. Post warming viability as parameter to find out freezing process in cell. DNA damage, change of RNA level and protein were potential side effects due to freezing, yet it did not always cause cell death (Lin and Tsai, 2012). Cattle oocyte freezing was able to induce degeneration of blastomere cell. Oocyte which was frozen and re-cultured showed that oocyte had apoptosis which was signed by cytoplasm condensation, chromatin condensation and hyper-segmentation, cytoplasm segmentation, and nucleolus formation which finally oocyte got dead (Gjørret et al., 2007; Glamocˇlija et al., 2005). Cryopreservation potentially induced apoptosis. Cryopreservation induced temperature stress so oxidative stress was likely to take place, therefore it affected composition of culture medium (Sudano et al., 2011).
Apoptosis in most cell types is accompanied by changes in Ca2+ homeostasis. During apoptosis, caspase-3 is mediated and will affect the type 1 inositol 1,4,5-trisphosphate receptor (IP3R1) to produce a C-terminal terminal fragment 95-kDa (C-IP3R1), which represents the receptor domain channel.
C-IP3R1 expression is not known to definitely contribute to Ca2+ levels, mis-management or decreased competence of oocytes. From several studies the expression of C-IP3R1 in oocytes decreases the content of Ca2+ endoplasmic reticulum (ER), though, because C-IP3R1 is rapidly degraded at this stage, the expression does not interfere with the development of pre-implantation embryos. CIP3R1 expression in oocytes increases the fragmentation associated with apoptosis. Endogenous IP3R1 is needed for aging apoptosis, because the regulation that is prevented prevents the fragmentation and expression of C-IP3R1 in oocytes. The expression of C-IP3R1 in the germinal vesicle (GV) oocyte produces a constant level of protein, decreases the ER’s ability to release Ca2+ that occurs during maturation, damages the oscillation ability of mature Ca2+ oocytes and potential activation. Ca2+ homeostasis and ER Ca2+ bond was needed during the oocyte maturation process (Zhang and Fissore, 2014).
High intensity of Caspase 3 in oocyte post vitrification was as a sign that oocyte had apoptosis (Figure 2). Several factors could cause apoptosis in oocytes. Oocytes was susceptible to different types of injuries following cryopreservation. The freezing technique used for cryopreservation and the physiological quality, chilling sensitivity, plasma membrane permeability, tolerance for osmotic stress, developmental stage, and species of the oocytes (Vajta G and Kuwayama, 2006). Activation of apoptotic processes mediated by caspases, as defined caspase activity assays, was a critical mechanism responsible for the disruption of oocyte capacity for cleavage and subsequent development (Roth and Hansen, 2004). So that, examine the intensity of apoptosis, maturation rate, fertilization rate of frozen oocytes needed the next study.
Ovaries consist of numerous follicles, oocytes, and granulosa cells in different stages of development. Many of these follicles will undergo an apoptotic process during the lifetime of the animal. By using proper tissue preparation methods, the events within the whole ovary can be observed by using Confocal Laser Scanning Microscopy. Caspase 3 in the follicle was detected as clusters of intensely stained granulosa cells located in close proximity to the oocytes. The proper use of the spectra from these fluorescence molecules is the foundation for high quality morphological images of apoptosis. By sequentially imaging the two probes with a 488 nm laser, there was a reduction in fluorescent cross talk and an increase in image quality (Zucker and Jeffay, 2006.
Conclusion
Caspase 3 intensity of frozen oocyte post vitrification was higher than that of fresh oocyte.
Acknowledgements
We would like to thank Universitas Airlangga through Post Graduate Program and Buraue of Research and Innovation that have funded this research from mandatory research scheme year 2019.
Conflict of Interest
Authors declare that they have no conflict of interest.
Authors Contribution
All authors contributed equally to the manuscript.
REFERENCES






