Advances in Animal and Veterinary Sciences
Review Article
Pathogenic Potential and Global Epidemiology of Bluetongue Virus that Cause Infection in Ruminants
Rana Haider Ali*, Suleman Irfan, H.M. Usman Siddiq, Toqeer Ahmed, Sami Ullah
Department of Microbiology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Abstract | Bluetongue is an infectious disease of ruminants. The etiological agent is Bluetongue virus that has double stranded RNA genome. Culicoides biting midges perform as a vector for virus spread. There are twenty seven bluetongue virus serotypes present in world. Bluetongue disease mainly affects sheep, goat, cattle, and camelids species. Bluetongue is a viral, non-contagious and infectious disease caused by a virus of the genus Orbivirus, transmitted by a hematophagous vector of genus Culicoides, to wild and domestic ruminants, primarily to sheep which is the most susceptible species. It is caused by link of endemic with climate conditions, with humidity and high temperatures. Economic loss is directly associated to abortion, loss of milk, death and meat production, weight loss and indirectly by restriction on export of the animals and their by-products. This article describes brief overview of bluetongue virus followed by its pathogenic potential and then its vector distribution and global epidemiology of its different serotypes.
Keywords | Bluetongue, Bluetongue virus (BTV), Serotypes, Epidemiology, Ruminants
Received | May 16, 2020; Accepted | August 21, 2020; Published | September 20, 2020
*Correspondence | Rana Haider Ali, Department of Microbiology, University of Veterinary and Animal Sciences, Lahore, Pakistan; Email: [email protected]
Citation | Ali RH, Irfan S, Siddiq HMU, Ahmed T, Ullah S (2020). Pathogenic potential and global epidemiology of bluetongue virus that cause infection in ruminants. Adv. Anim. Vet. Sci. 8(s2): 1-6.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s2.1.6
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Ali et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Bluetongue is an infection of small and large ruminants triggered by Bluetongue virus (Ayanur et al., 2016; Becker et al., 2020). Virus is spread by bite of an insect vector among the domestic and wild ruminant species. Disease is found in subtropical, tropical and temperate regions worldwide (Pandrangi, 2012). Bluetongue is counted as notifiable disease according to classification stated by OIE due to its economic significance (OIE, 2008). Notifiable diseases are generally the diseases that have the potential of communicability and swift spread. These diseases are frequently significant regarding public health concerns and socioeconomic impact (Alexander et al., 1996).
Bluetongue virus belongs to Reoviridae family and Orbivirus genus. BTV is transmitted to the ruminants by biting midges of the Culicoides genus (Sbizera et al., 2019). It is non developed virus having icosahedral symmetry. Diameter of this virus is 90 nanometers and it has 3 layers of capsid. Genome contains double stranded RNA that has ten segments while each segment codes for different protein. Segments code for 4 non structural and 7 structural protiens (NS1 to NS3, NS3A and VP1 to VP7 respectively). VP2 and VP5 protiens make the outermost layer of virus (Roy and Noad, 2006). VP2 Protein is involved in determining the virus serotype because it is responsible for binding to the receptors, stimulating host-specific immune response and haemagglutination. The second major protein is VP5 that is responsible for interaction with host cell endosomal membrane and responsible for playing a little role in provoking antibody response against virus (Roy, 2008). The VP7 protein forms the middle or inner capsid called core (Nason et al., 2004). VP7 protein is main determinant of serotype specificity and the target for most of the serological tests for identification of antibody against Bluetongue virus. Other proteins VP1, VP3, VP4 and VP6 form the innermost layer, plays role in the replication and transcription of genome (Anthony et al., 2007; Schwartz-Cornil et al., 2008). The non structural proteins control numerous other vital functions such as virus maturation, replication, and export of new virus progeny from the infected cell (Ratinier et al., 2011).
Bluetongue disease cause significant economic losses of livestock (van der Sluijs et al., 2016). Economic losses are due to low trade rate linked with bluetongue disease outbreaks and high morbidity and mortality (Rojas et al., 2019). The losses are direct (abortion, decreased milk production, death, loss of weight and efficiency of meat production) and mostly indirect as a result of restrictions to the export of semen, live animals and some products such as bovine fetal serum (Acevedo et al., 2016).
Pathogenesis of blue tongue virus
Infected midge transmits the bluetongue virus by bitting the susceptible animal. From the skin, the virus is transported to local lymph nodes via host dendritic cells (Hemati et al., 2009). Virus then infects endothelial linings of the blood vessels, dendritic cells and mononuclear phagocytic cells (Mahrt and Osburn, 1986; Barratt-Boyes et al., 1992; Hemati et al., 2009). This results in tissue infarction, necrosis and vascular thrombosis that leads to hemorrhage (Pini, 1976; Mahrt and Osburn, 1986; Verwoerd and Erasmus, 2004). Subsequently, the virus spreads to the other parts of the body through blood circulation and primary viremia is induced. From the blood, virus is transmitted to secondary organs such as spleen, lungs and lymph nodes (Sánchez-Cordón et al., 2010). In early viremia, virus associates itself with the blood components. On following stages, the virus particles attach and are sequestered in the invaginations of the erythrocyte membrane (MacLachlan, 2004). The tissue damage results in the clinical manifestation of disease as serous or sometimes bloody discharge from nose, malaise, severe pulmonary and facial oedema, ulcers in mouth and lameness (Verwoerd and Erasmus, 2004; Maclachlan et al., 2009). BT virus infection leads to cell apoptosis and necrosis (Mortola et al., 2004). p38MAP kinase is activated that enhances the vascular permeability. This initiates the release of cyclooxygenase-2, IFN-I, IL-1, IL6, IL-8 and TNFα that cause the enhanced production of thromboxane and prostacyclin in blood plasma resulting in the excessive inflammatory response. This inflammatory response is involved for the development of clinical signs of the disease caused by damage to the infected animal cells and tissues (Drew et al., 2010). The mortality rate in sheep is ranged between 2 to 30 percent (Verwoerd and Erasmus, 2004) but occasionally can be up to 70 percent (Gambles, 1949). The recovery of the infected animals can be prolonged and during that period animals can suffer reduced fertility, wool quality and milk production (Verwoerd and Erasmus, 2004; Maclachlan et al., 2009). These cause the direct economic loss due to mortality and morbidity in susceptible and infected animals. Furthermore, the restrictions on animal trades internationally and the germ plasm cells from the BT endemic countries cause significant indirect economic losses (MacLachlan and Osburn, 2006). The cost for surveillance, vector control strategies, mass vaccination of susceptible animals and treatment of infected animals also contributes to economic loss (Wilson and Mellor, 2008). Bluetongue virus infects ruminant species, including sheep, cattle, goat, camelid and deer. The virus usually remains asymptomatic and subclinical in cattle and goats. Sheep and deer are more susceptible to bluetongue disease with development of clinical disease (Elbers et al., 2008). Clinical signs developed in infected animals include fever, lameness, cattaral inflammation of hooves and lips, conjunctivitis, hyperaemia and salivation (Darpel et al., 2007). In severe cases, it can also induce abortion in the infected pregnant animals. Even after the disease recovery of animal, it may manifest different long lasting secondary effects. These include reduced milk production, wool breakage, less weight gains and sometimes sterility in animal (Wilson and Mellor, 2009). The virus can trigger an acute infection in an immunologically naïve population where bluetongue virus is not usually prevalent. For example, in the Europe BTV infection of serotype 8 caused clinical signs of disease in both goat and cattle despite of the fact that disease is usually sub clinical in the cattle. The clinical signs observed in cattle included conjunctivitis, oral mucosal congestion, discharge from eyes, necrotic lesions and ulcers and odema of lips, tongue and ocular discharge, ulcer formation and necrosis on tongue and lips (Dal Pozzo et al., 2009). The clinical signs observed in goats are facial odema, odema of lips, erythema of skin and udder and discharge from nose (Dercksen et al., 2007). Cattle generally plays the role of a virus amplifying host in BTV endemic areas (Barratt- Boyes et al., 1992).
Vector distribution
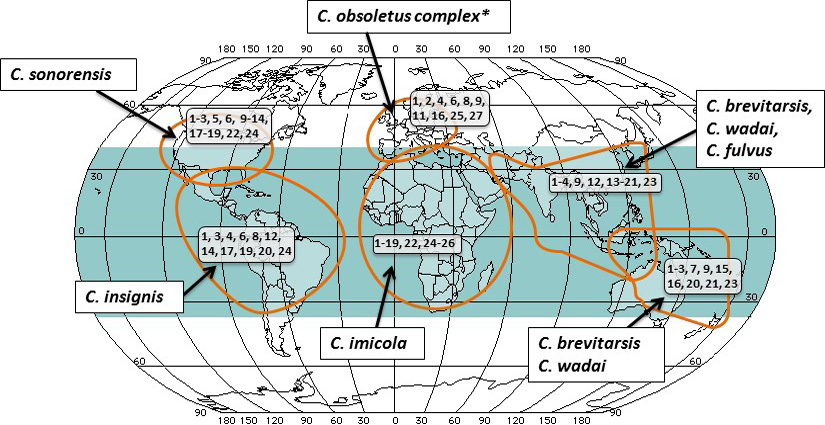
Differences in activity, distribution and virus transmission competence of adult Culicoides populations can determine if an area is disease free, endemic or seasonally disease free. In spite of existence of more than two thousand species of the Culicoides midges, only a few are known to be competent vectors for the bluetongue virus. Natural barriers such as deserts and oceans affect the free movement of the insects and BTV between different geographical areas where the competent vector species exist. The most widespread of known vector of virus is C. imicola, with the habitat extending from the most parts of Southern Europe, Mediterranean, Africa, south and west Asia and east Asian countries, including Vietnam, Laos and China. Recently, C. imicola has been found in new areas of the southern Europe, coinciding with spread of bluetongue virus to these regions (Wilson and Mellor, 2009; Carpenter et al., 2013). In the endemic areas where the C. imicola has not been found, including the Northern Europe, Americas, southeast Asia, northern China and Australia (Mellor et al., 2009). Figure 1 depicts the relationship between major Culicoides vectors, their predominant geographic location, and existence of the Bluetongue virus serotypes (indicated within black boxes).
Global distribution and epidemiology of BTV
Bluetongue disease is a considerable socioeconomic concern and of great significance for international trade of animal products and animal. In past, bluetongue endemic areas were considered those between latitudes 40 °N and 35 °S, but it has spread far beyond this traditional range. This disease has numerous serotypes and these serotypes are present in the complex network of serological cross relationships varying from partial to no protection between the heterologous strains (Cappai et al., 2019).
Bluetongue virus infection of ruminants and vector Culicoides insects is enzootic throughout temperate and tropical regions of the world however, drastic regional changes in global distribution of Bluetongue virus infection have occurred particularly in the Europe since 1998. Various new Blue tongue virus serotypes also have been discovered since 1998 in the south eastern United States, evidently encroaching from the adjoining Caribbean ecosystem and new serotypes of virus have been detected in other historically enzootic regions of the world including the Australia and Middle East. (Maclachlan, 2011). The global dissemination of bluetongue Virus corresponds to variations in the climate and worldwide distribution of the Culicoides vector. Climate change is the possible cause of this change particularly in the Europe. There are 27 bluetongue virus serotypes circulating worldwide (Maan et al., 2012; Jenckel et al., 2015). Twenty two serotypes are endemic and co circulate in the southern Africa (Zulu and Venter, 2014). Many serotypes (BTV-1, 2, 4, 6, 8, 9, 11 and 16) entered the Europe since 1998. BTV-10, 11, 13 and 17 are present throughout North America. Many serotypes such as 1, 3, 5, 6, 9, 12, 14, 19, 22 and 24 have found to exist in southeastern parts of the United States after 1998 (Maclachlan et al., 2013). The virus was detected in 12 countries of Europe between 1998-2005 that caused the death and culling of over one million sheep (Purse et al., 2005). In Australia, ten serotypes (1, 2, 3, 7, 9, 15, 16, 20, 21 and 23) of BTV were reported (Boyle et al., 2012). Serotype BTV-6 was reported from Germany and Netherlands while, BTV-11 was recognized from Belgium in year 2008 (Maclachlan and Guthrie, 2010). In 2008, new serotype BTV-25 was isolated in Switzerland. In Kuwait serotype 26 was identified in the year 2010 (Maan et al., 2012). Twenty two different serotypes of BTV were observed from all over the India (Shafiq et al., 2013). During the year 2003, 90% of the seroprevalence was reported during a serological survey in cattle was performed in Turkey and 23 provinces in Anatolia. BTV serotypes 4, 9 and 16 were observed circulating in Anatolia, Turkey. To device the constrains on disease spread different strategies were applied such as constraints on animal whereabouts, disease surveillance and live vaccination of animals with locally produced live attenuated vaccine against the serotype 4 (Mellor and Wittmann, 2002). In a study BTV serotypes 1, 6, 8, 11, 14, 25, 27 were reported in Western and Central Europe (Jenckel et al., 2015; Martinelle et al., 2016). Table 1 describe an up to date list of distribution and epidemiological pattern of various serotypes of Bluetongue virus.
A recent study conducted in Punjab, the overall prevalence was observed to be 28.81% in the small and large ruminants. The highest prevalence found in province was in goats followed by buffalo then sheep and cattle. The risk factor analysis showed that buffalo of Nili Ravi breed and detected with the presence of vector were at the higher risk of being seropositive for virus. Both buffalo and buffalo with history of the abortion were known to be much likely carrying bluetongue disease. Real time PCR based serotyping showed detection of serotypes 4, 6 and 8 in sheep, 2, 8 and 16 in buffalo, 2, 6, 8 and 11 in goats and only serotype 8 in cattle (Sohail et al., 2019). Another recent study conducted in Balochistan showed overall seroprevalence of bluetongue virus was found to be 47.26% in goats and sheeps. Seroprevalence was more in goats than sheep. The Serotype 8 was again the most prevalent serotype in the Balochistan province followed by an equal prevalence of the serotypes 2 and 9 (Sohail et al., 2018).
Conclusion
Bluetongue is non-contagious arthropod borne viral disease of wild and domestic ruminants. Bluetongue is one of the most important viral diseases of livestock, causing great economic losses mainly due to its effect on trade. Although the disease is comparatively easy to control in completely and seasonally free areas, highly concerted efforts are needed to control it in the endemic areas. The condition is complicated by the presence of multiple serotypes, genotypes and topotypes of bluetongue virus, transmission by insect vectors and involvement of asymptomatic hosts. Because different geographical regions present unique conditions in terms of hosts, viruses, vectors and climatic conditions, it is important that continuous monitoring of circulation of bluetongue virus be carried out, not only to classify different strains of bluetongue virus circulating in the specific areas but also to make the relevant diagnostics and vaccines. In view of the threat of the virus entering new regions, surveillance and epidemiology in endemic areas, which act as the source of virus to neighbouring sink areas, is of great significance.
* C. obsoletus complex comprises of numerous other closely linked species of the midges. This specific complex and its several species is not all inclusive to European region, but is the primary species in addition to C. imicola which present in Mediterranean area.
Reference: Adapted from Tabachnick W.J., University of Florida, Institute of Food and Agricultural Sciences (UF/IFAS). (2008). Bluetongue. Retrieved from http://edis.ifas.ufl.edu/pdffiles/IN/IN76800.pdf.
Table 1: Describe an up to date list of distribution and epidemiological pattern of various serotypes of Bluetongue virus.
| Serotypes | Region | Year of discovery | Reference | |
| 2, 6, 8, 9 | Pakistan | 2019 | ||
| 1, 4, 8 | Morocco | 2004 to 2015 | ||
| 27 | Corsica (France) | 2014 | ||
| 26 | Kuwait | 2010 | ||
| 25 | Switzerland | 2008 | ||
| 11 | Belgium | 2008 | ||
| 6 | Germany and Netherland | 2008 | ||
| 2 | Bulgaria | 2000 | ||
| 1, 2,3,4, 6, 9, 10, 12, 16,17,18,21,23 | India | 1967 to 2014 | ||
| 1, 3, 5, 6, 9, 12, 14, 19, 22, 24 | South eastern United States | 1998 | ||
| 10, 11, 13, 17 | North America | 1998 | ||
| 1, 2, 4, 6, 8, 9, 11, 16 | Europe | 1998 | ||
| 20 | Australia | 1976 | ||
| 10 | South West Spain and Portugal | 1956 to 1960 |
(Lopez and Botija, 1958; Manso-Ribeiro and Noronha, 1958; Gorman, 1990). |
|
Acknowledgements
The authors wish to acknowledge the contribution and efforts of Dr. Hakeem Ullah for his critical review of manuscript draft.
Authors Contribution
All the Authors contributed equally in the preparation of manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References