Advances in Animal and Veterinary Sciences
Research Article
Production and Characterization of Polyclonal Antibodies for Diagnosis of Pregnancy in Cattle
Yeni Widyaningrum1*, M. Luthfi1, Dian Ratnawati1, Lukman Affandhy1, Aulanni’am2, Agung Pramana Warih Marhendra2, Yuli Arif Tribudi3
1Indonesian Beef Cattle Research Station, Pasuruan, Indonesia; 2Department of Biology, Faculty of Mathematics and Natural Sciences, University of Brawijaya, Malang, Indonesia; 3Faculty of Agriculture Universitas Tanjungpura, Pontianak, Indonesia.
Abstract | Pregnancy-specific protein B (PSPB) is produced by binucleate trophoblast cells in the bovine placenta around the time of implantation. We produced anti-PSPB polyclonal antibodies to detect PSPB in the serum of Peranakan Ongole (PO) pregnant cows. Six New Zealand White (NZW) Rabbits were immunized with 40-60 kDa protein in combination with adjuvants for antibody production. Sera of experimental animals (n = 30) were taken from the auricular vein and subjected to the ELISA test. The highest antibody titer was used to test the specificity of antibodies using the dot blot method. Antibody titer was observed during the first week, while the highest antibody titer (OD = 0.3755) was found during the 3rd week of bleeding, which decreased gradually during the subsequent weeks. The sensitivity test showed an ability of anti-PSPB to detect PSPB in the serum of pregnant cows from 90 days of gestation and as low as 1:120. The study concludes the potential utility of anti-PSPB polyclonal antibodies for the detection of pregnancy in cows.
Keywords | Cattle, Anti-PSPB, Polyclonal antibodies, Diagnosis of pregnancy
Received | February 25, 2020; Accepted | June 22, 2020; Published | July, 18, 2020
*Correspondence | Yeni Widyaningrum, Indonesian Beef Cattle Research Station, Pasuruan, Indonesia; Eamil: [email protected]
Citation | Widyaningrum Y, Luthfi M, Ratnawati D, Affandhy L, Aulanni’am, Marhendra APW, Tribudi YA (2020). Production and characterization of polyclonal antibodies for diagnosis of pregnancy in cattle. Adv. Anim. Vet. Sci. 8(8): 833-838.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.8.833.838
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Widyaningrum et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The success of reproductive management is characterized by a high rate of mating, pregnancy, and birth in cattle farming. Diagnosis of pregnancy, in this regard, plays a pivotal role in profitable animal husbandry, especially for production animals such as cattle, goats, and sheep. Detection of pregnancy at an early stage allows identification and treatments of subsequent complications and, therefore, may play a key role in management-related interventions to improve the reproductive efficiency of commercial farms (Bekele et al., 2016; Fricke, 2010). Hence, an appropriate, rapid, and accurate method for diagnosis of pregnancy is much needed to ensure the success of pregnancy.
Traditional rectal palpation is probably the most commonly used method for the diagnosis of pregnancy in cattle until now. However, it can be performed as early as 60 days after artificial insemination (AI) (Patel et al., 2016) and requires assisted reproductive technology (ART) (Lucy et al., 2007). Therefore, there is a need for alternative methods that can be used for early diagnosis of pregnancy in cattle in less than one month of gestation. For the subject matter, there are several procedures in practice, but each one of them has associated limitations. For example, the measurement of endocrine changes (progesterone hormone) can be used for early diagnosis of pregnancy; however, it is not cost-effective and needs associated laboratory infrastructure (Resee et al., 2016). The radioimmunoassay (RIA) method uses radioactivity and, therefore, may induce radiation effects. The immunologic diagnosis is another alternate for early diagnosis of pregnancy since, after mating or AI, it can detect pregnancy in less than 20 days. In this regard, one of the commonly used methods is anti-pregnancy-specific protein B (PSPB) antibodies (Hafez, 2000).
A specific antigen, bovine pregnancy-associated glycoprotein (BPAG), belongs to a family of inactive aspartic proteinases (Xi et al., 1991) containing 22 genes situated on chromosome 29 (Telugu et al., 2009), of which the pregnancy-specific protein B (PSPB) is the first member to be discovered (Butler et al., 1982). The PSPB is a protein substance secreted by blastocyst during implantation and subsequent pregnancy, and found in the binucleate cells of the ruminant trophectoderm (Sasser et al., 1986). Furthermore, the bovine PSPB fraction is characterized as glycoproteins and has relative molecular weight mass 47-53 kDa (Butler et al., 1982). A presence of PSPB in the sera of post-mating cows can be considered pregnant during embryo implantation period (Hafez and Hafez, 2000). The BPAG can be detected in the maternal circulation as early as 15 day after conception (Green et al., 2009). The production of antibodies against the purified proteins can be used to detect the presence of the protein in the bovine peripheral circulation. The BPAGs such as PSPB have been employed for the detection of early pregnancy in cattle (Howard et al., 2007; Breed et al., 2009; Piechotta et al., 2011). Because these glycoproteins are specific to the placental tissue, it is possible to use BPAGs in the maternal circulation as an indicator of pregnancy (Ga´bor et al., 2007). The findings reported by Sasser et al. (1986), Howard et al. (2007), and Ergene et al. (2018) concluded the PSPB test to be more accurate than the traditional rectal palpation for early diagnosis of pregnancy. With this background, we conducted this study to produce anti-PSPB polyclonal antibodies for the detection of PSPB in the serum of Peranakan Ongole (PO) pregnant cows.
MATERIALS AND METHODS
Location of Study
This study was carried out at the Indonesian Beef Cattle Research (BCR) Laboratory for three months. Protein analysis was performed at the Laboratory of the Faculty of Veterinary Medicine, Universitas Brawijaya.
Research materials
Thirty Peranakan Ongole (PO) cows were used for the collection of blood sera and further analysis. These were divided into three groups and diagnosed pregnant after 30, 60, and 90 days of artificial insemination. Six New Zealand white rabbits (Oryctolagus cuniculus; male; 6 months of age) were used for anti-PSPB production. They were fed with concentrate and forages, and drinking water was given ad libitum.
Sample collection and PSPB isolation
This study was conducted following the guidelines for the care and use of animals from the Indonesian Agency for Agricultural Research and Development (Balitbangtan/Lolitsapi/RM/03/2016). Sera were taken from the auricular vein and collected in venoject tubes (Terumo Corporation, Tokyo, Japan). PSPBs were extracted from the sera as per the method described previously (Aulanni’am et al., 2012). Briefly, blood samples were added with 1 ml PBST-PMSF and centrifuged (6000 rpm for 15 minutes). The supernatant was taken and stored at -800C until it was analyzed. The protein samples were precipitated by the addition of absolute ethanol (1:1), vortexed, incubated overnight at -20oC, and centrifuged (12000 rpm for 10 minutes at 4 oC. The supernatant was removed; the precipitate was dried, added with Tris-HCL (1:1), and stored at 80 oC until it was analyzed. Furthermore, identification and purification of the isolated protein were performed using the SDS PAGE. The isolated protein (2 µL) was dropped on the pedestal submicroliter cell that had been cleaned with ddH2O. Protein concentration was determined using a NanoDrop™ 1000 Spectrophotometer, and the absorbance was instantly displayed on a monitor screen.
SDS-page
The SDS-PAGE used in this study was performed in accordance with Aulanni’am (2005). Briefly, protein samples were mixed with reducing sample buffer (RSB) (1:1), heated at 100ºC for 5 minutes, and incubated at 20ºC. For gel production, 12% separating gel (made up of 10% SDS, 30% polyacrylamide, 10% APS, 1 M Tris pH 8.8, Aqua bidest, and TEMED) and stacking gel (made up of Bis-acrylide, 10% SDS, 1 M Tris pH 6.8, APS 10%, Aqua bidest, and TEMED) were made. The gel plates were attached to the electrophoresis chamber (Mini-PROTEAN Tetra Cell, Bio-Rad). The upper and lower tanks of the chamber were filled with a running buffer. Then 10-20 μL protein samples and molecular weight (MW) protein markers were loaded on the gel. The gel was run at 20 mA constant current for 40-50 minutes, until the tracking dye reached approximately 0.5 cm above the bottom of the gel. Then gel was stained with Coomassie Brilliant Blue G 250 (Bio-Rad Laboratories, USA) for an hour and photographed using the GelDoc documentation system (Bio-Rad). Moreover, molecular weight of particular protein samples was determined by comparing the band patterns between protein samples and MW protein markers.
Anti-PSPB production and purification
Anti-MMP-3 antibodies were produced in the rabbit. Induced by PSPB, rabbit anti-PSPB antibodies (IgG) were purified using 50% saturated ammonium sulfate (SAS) as per the method described previously (Aulanni’am et al., 2012) (Table 1).
Table 1: Immunization and Ig G extraction procedure.
| Week | Procedure | Adjuvant |
| 1 | First immunization (Bleeding pre-immune) | CFA |
| 3 | Second immunization (Booster 1) | IFA |
| 4 | Bleeding 1 | - |
| 5 | Bleeding 2 | - |
| 6 | Bleeding 3 | - |
| 7 | Bleeding 4 | - |
| 8 | Bleeding 5 | - |
| 8 | Third immunization (Booster 2) | IFA |
| 9 | Bleeding 6 | - |
| 10 | Bleeding 7 | - |
| 11 | Bleeding 8 | - |
| 12 | Bleeding 9 | - |
| 13 | Bleeding 10 | - |
CFA: Complete Freund’s Adjuvant; IFA: Incomplete Freund’s Adjuvant.
Anti-PSPB immunogenicity test using indirect ELISA
Using an indirect ELISA method, anti-PSPB antibody titers were measured according to the previously described protocol (Eivazi et al., 2015).
Immuno-dot analysis
The study proteins were coated on nitrocellulose (NC) membrane. Membranes were added with antigen membranes and blocked with 5% PBS skimmed milk and incubated for two hours. The membranes were washed thrice with PBS-T for each of 3 minutes. The membranes were incubated in diluted sera with 1% PBS skim milk for an hour. The membranes containing antigen-antibody a complex were washed thrice and incubated with secondary antibodies (Anti-Rabbit IgG Conjugate AP) for an hour. The membranes were washed thrice again, added with alkaline phosphatase (AP) substrate (0.21 mg/mL Nitro Blue Tetrazolium and 0.42 mg/mL Bromo-4-chloro-3-indoxyl-phosphate in a Tris buffer solution), and incubated for 30 minutes. Finally, the membranes were washed with distilled water, dried, and the formed color density was analyzed as described previously (Aulanni’am et al., 2012).
Data analysis
Data obtained were analyzed using descriptive statistics.
RESULTS AND DISCUSSIONS
Protein concentration determination
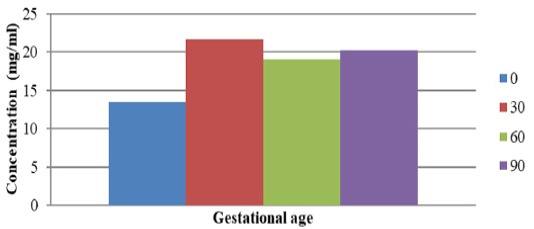
The production of anti-PSPB antibodies in rabbits were assessed through the ELISA test. The concentrations of serum protein in pregnant cows during 1 (A), 2 (B), and 3 (C) months of gestation, and in non-pregnant cows (O) are shown in Figure 1. The concentration of serum protein samples was 13.5 mg/mL for non-pregnant (O) cows while it was found to be 21.7 mg/mL (A), 19.1 mg/mL (B), and 20.2 mg/mL (C) for pregnant cows. This indicated a variation observed in serum PSPB concentration among pregnant and non-pregnant cows, which was similar to the findings of some previous sdudies. Dhakal et al. (2017) observed low (0.24 ± 0.025 ng/mL) and high (30.46 ± 12.67 ng/mL at day 48 after AI) levels of serum PSPB concentration in non-pregnant and pregnant Nepalese buffalo, respectively, while Abdulkareem et al. (2011) and El-Battawy (2009) found 3.89 ± 0.53 ng/mL at 32-34 days after AI and 4.48 ± 0.92 ng/mL at day 28 after AI, respectively. Furthermore, 6480 ng/mL PSPB concentration isolated from cotyledons was observed in Friesian Holstein pregnant cows (Samik, 2010). Highest PSPB concentration in pregnant cows during 1 month of gestation, as observed in this study, was similar to findings reported by Ergene et al. (2018). They reported that PSPB in bovine plasma was detected between 15 and 22 days of pregnancy after AI. In most studies, PSPB test was found to be useful for the early detection of pregnancy in cattle. However, good results may be obtained when the PSPB test is performed after day 30 post-AI, because glycoprotein is synthesized in the binuclear trophoblastic cells of the placenta (Szenci et al. 1998).
Specific proteins band profiles of PO pregnant cows
Characterization of pregnancy-specific protein B (PSPB) in pregnant- and non-pregnant cows was made through the SDS-PAGE method (Figure 2). A protein band profile indicating the molecular weight of each type of protein in sera was obtained. A total of nine protein bands were observed in gel electrophoresis, which showed a thicker color than others. The molecular weights of these proteins were measured using linear equations (Table 2).
The protein band profiles are presented in Figure 2. The results showed that proteins, ranging from 45 kDa to 60 kDa, were observed exclusively in the sera of pregnant cows. PSPB is the first member of aspartic peptidases to be discovered in the ruminant placenta with relative molecular weight of 45-53 kDa (Butler et al., 1982). Later, Zoli et al. (1992) used a radioimmunoassay (RIA) to develop a measurement of PSPB in the circulation of cattle and described the first establishment of a protein marker for early diagnosis of pregnancy in cattle. The results of this study are comparable with those of several previous studies which also found PSPB in the serum of pregnant cows with molecular weights up to 60 kDa (Sasser et al., 1986; Xie et al., 1991; Huang et al., 1999; Samik, 2010; Balhara et al., 2013; Wallace et al., 2015).
Table 2: Protein molecular weight determination.
| Sample Band n- | Molecular Weight (kDa) |
| 1 | 158.36 |
| 2 | 107.71 |
| 3 | 83.17 |
| 4 | 70.80 |
| 5 | 54.14 |
| 6 | 26.56 |
| 7 | 21.53 |
| 8 | 11.8 |
| 9 | 9.3 |
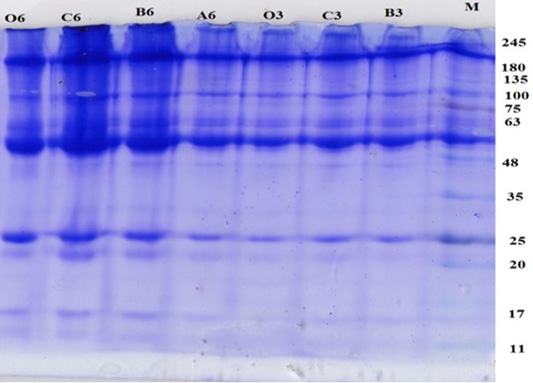
Figure 2: Protein band profile in PO pregnant cows with gestational age of 1, 2 and 3 months and control. M: Maker; A: 1 months of gestation, B: 2 months of gestation; C: 3 months of gestation.
The protein band color for pregnant cows less than 30 days of gestation (A6) was thinner compared to that for pregnant cows who were at 60 and 90 days of pregnancy. This may correspond to the concentration of specific proteins in sera, which become increased with an increased period of gestation. Higher production of particular proteins, particularly at 2 to 3 months of gestation, is ascribed to a higher number of trophoblast cells (Hasrati, 2001; Reese et al., 2016).
Determination of polyclonal antibody titer using ELISA
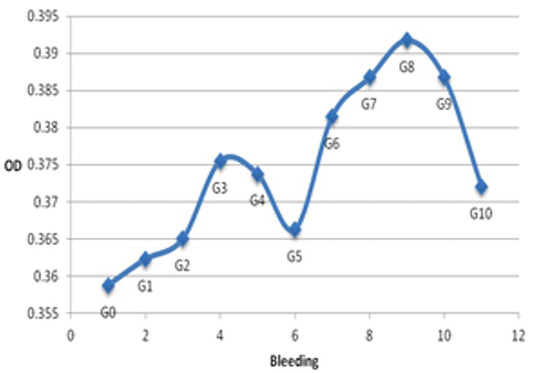
Harvested from NZW rabbit immunization, polyclonal antibody titers were determined by ELISA that were able to recognize bovine IgG (Figure 3). On the other hand, polyclonal anti-IgG (PAb) antibodies interacted with bovine IgG with very high specificity and affinity. The concentration of anti-PSPB antibodies in the pre-immune serum was the lowest [optical density (OD) of 0.35875] (Figure 3). This is because pre-immunes serum was collected from NZW rabbits before immunization and, therefore, did not produce anti-PSPB antibodies. An increase in antibody titers began from the first week of bleeding (OD of 0.36225). This is because considered as a foreign subject, PSPB triggered an immune response by binding to B cells. The highest antibody titer was observed at the 3rd week of bleeding (0.3755k), which deceased gradually in the subsequent weeks. Similarly, as reported by Samik (2010), the antibody titers began to increase from day 0 (before immunization, OD= 0.179 ± 0.010) to the second week after booster (OD= 2.256 ± 0.4842). After the first booster, an increase in antibody titer was attributed to the presence of PSPB antigen memory in rabbits, which further stimulated antibody production from the first week of bleeding. Immunoglobulin G was detected in the serum approximately 67 days after exposure to antigens (Baratawidjaja and Rengganis, 2009). However, after the third immunization, the production of antibodies increased gradually and reached its highest peak at the 8th week of bleeding (OD= 0.39175), which decreased steadily until the 10th week of bleeding. Booster activates B memory cells to recognize the antigen entering the body and responds by producing specific antibodies. Antibody titers were observed to be higher at the third immunization (second booster) than that found at the first and second immunization. This is because the amount of memory B cells and plasma B cells are higher than those after the first booster. Siregar (2006) stated that, upon the second booster, the secondary immune response leads to a faster proliferation of memory B cells and generates a higher concentration of antibodies.
Analysis using indirect ELISA showed that PSPB antibodies produced in the serum of pregnant cows at different intervals of gestation were immunogenic because they can stimulate immune systems by providing polyclonal antibodies against antigens. Thus, the production of polyclonal antibodies can be used as a rapid, precise, and accurate tool for early diagnosis of pregnancy in bovine. Polyclonal antibodies were used in this study because they had better sensitivity than monoclonal antibodies and, therefore, can detect various epitopes present in a single antigen (Sadeghi et al., 2018). According to Eivazi et al. (2015), polyclonal antibodies can form more antigen-binding sites and, therefore, can result in a better sensitivity than monoclonal antibodies.
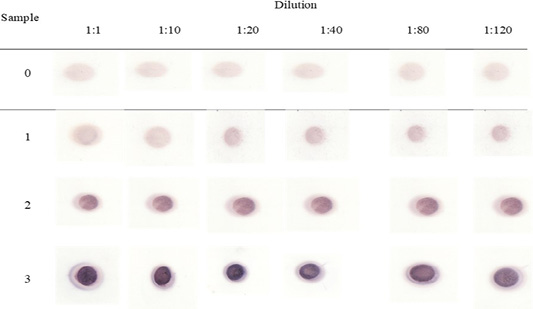
Figure 4: PSPB dot blott assay with antibodies.
Sensitivity test for anti-PSPB antibody using the dot blot method
The sensitivity test was performed using the dot blot method in various dilution factors, and the results are shown in Figure 4. Degradation and density of purplish color indicated the number of bonds between specific proteins and specific protein antibodies. The results indicated that, at a dilution of 1:1, anti-PSPB had the highest color density, and it was least at a dilution of 1:120. The decrease in color density at a dilution of 1:120 may correspond to the decreased concentration of antigens in the sera of pregnant cows and consequently decreased antibodies. However, at a dilution of 1:120, anti-PSPB was still able to recognize antigens in the sera of pregnant cows even at 30 days of gestation. The results of this study suggested that the bovine PSPB test gave a good sensitivity for early diagnosis of pregnancy as less as the 30th of gestation. The PSPB test was found to have good sensitivity (98.0%), specifity (97.1%) and accuracy (97.8%) for early diagnosis of pregnancy in dairy cows (Piechotta et al., 2011). Ergene et al. (2018) observed a lower sensitivity (93.8%) for bovine PSPB test on day 28 after AI. Szenci et al. (1998), using the bovine PSPB RIA test, observed a sensitivity of 92% from samples collected on both days 29 and 30, and 98.1% on days 33 and 34. In addition, Howard et al. (2007), using the bovine PSPB ELISA test, showed a sensitivity of 100% and a specificity of 87.8% from samples collected between days 30 and 36. In general, the present results indicated that rabbit anti-PSPB polyclonal antibodies can specifically recognize antigens in the sera of pregnant cows.
CONCLUSION
The study concludes that PSPB is a suitable marker for efficient diagnosis of pregnancy as less as the 30th day of gestation.
ACKNOWLEDGMENTS
The Indonesian Agency funded this study for Agricultural Research and Development (IAARD), Ministry of Agriculture, under a DIPA scheme (No. 648720.1806.105.001.051.1.2016).
AUTHORS CONTRIBUTION
Yeni Widyaningrum, M. Luthfi, Dian Ratnawati, Lukman Affandhy and Aulanni’am shared in the study design, collection of samples, development of methodology and manuscript revision. Agung Pramana Warih Marhendra and Yuli Arif Tribudi shared in laboratory work, interpretation of data and writing the manuscript.
Conflict of Interest
The authors have declared no conflict of interest.
REFERENCES