Advances in Animal and Veterinary Sciences
Research Article
Prevalence of Methicillin-resistant Staphylococcal Mastitis in Ruminants
Saoussene Smaali*, Fatima Boukazoula, Lamia Saadi, Imen Brahem
Faculty of Exact Sciences and Natural and Life Sciences. Larbi Tebessi University -Tebessa, Algeria.
Abstract | The objectives of this work are to study the prevalence of staphylococcal mastitis, evaluate the resistance profile of these strains to antibiotics and the frequency of methicillin-resistant staphylococci in order to better define an effective therapeutic and preventive strategy. Milk samples from 41 females (cow, ewe and goat) were collected aseptically, and in the first step bacteriological tests were performed, including isolation and identification tests, to study the sensitivity of isolated staphylococci to nine antibiotics, using the antibiotic susceptibility reference method (agar dilution). The results were interpreted according to the criteria of Veterinary recommendations of the antibiotic susceptibility testing committee of the French microbiology society 2018. The results of bacteriological analyses of milk showed a significant prevalence of methicilin-resistant staphylococci mastitis (19.45%), including 17.85% of CSN and 1.2% for MRSA. The study of the antibiotic resistance profile of these isolated staphylococcus strains showed the high resistance of tetracycline with a frequency of 75% for CSP and 25% for CSN. This during vancomycin remains the most active antibiotic (100%) for both types of staphylococci.
Keywords | Algeria, MRSA-L, CSP, CSN, Livestock
Received | September 09, 2019; Accepted | January 10, 2020; Published | March 25, 2020
*Correspondence | Saoussene Smaali, Department of Applied Biology, Faculty of Exact Sciences and Natural and Life Sciences. Larbi Tebessi University -Tebessa, Algeria; Email: [email protected]
Citation | Smaali S, Boukazoula F, Saadi L, Brahem I (2020). Prevalence of methicillin-resistant staphylococcal mastitis in ruminants. Adv. Anim. Vet. Sci. 8(4): 428-432.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.4.428.432
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Smaali et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The pathology of the mammary gland is one of the main diseases encountered in Algerian dairy farms (Bouzid et al., 2011). It is a multifactorial disease. The risk of its occurrence can be increased by chemical, physical or traumatic factors. Even after several control programs have been implemented, mastitis is still a high incidence disease worldwide, resulting in very significant economic losses due to a decrease in the quality and quantity of milk production, an increase in the cost of therapeutic protocols and veterinary services services (Oviedo-Boyso et al., 2006).
The large use of antibiotics is the most common strategy of control of mastitis especially during the dry period by intramammary administration of long acting antibiotics in tubes has played a major role in the development of bacterial antibiotic resistance. This resistance to ATB is mainly due to the high consumption of antibiotics for their treatment but also for their prevention (Angoujard, 2015).
The emergence of multiresistant bacteria (BMR) will increasingly confront clinicians with real therapeutic impasses (Sanders et al., 2011). In addition, the transmission of this type of bacteria from animals to humans is possible either through direct contact or via the food chain from food and animal products contaminated by these bacteria (Petinaki and Spiliopoulou, 2012).
Staphylococcus is the germ most involved in mastitis in Algerian farms. The study of the epidemiology of this species has evolved considerably over time, due to its importance in veterinary and human medicine. The increase in infectious episodes caused by this multidrug-resistant pathogen, particularly strains of methicillin-resistant S. aureus (MRSA) of animal origin, especially those associated with livestock (MRSA-L), is intriguing to the scientific community and health professionals. In particular, since their zoonotic potential has been demonstrated (Petinaki and Spiliopoulou, 2012; Verkade and Kluytmans, 2013).
Despite the definite danger that these MRSA-L strains represent to human health, their existence is useful for science by serving as a study model to better understand host-pathogen interactions and to identify virulence factors and predisposing factors for the development of clinical infection. This improved knowledge is likely to open up new perspectives for treatment and control programmes in order to regain control over this formidable pathogen that causes harm in terms of profitability on farms and sometimes threatens people’s lives.
It is in this context that our study, whose objectives are to study the prevalence of staphylococcal mastitis, evaluate the resistance profile of these strains to antibiotics and the frequency of staph methicillin resistance in order to better define an effective therapeutic and preventive strategy.
Materials and Methods
The study was carried out on milk samples from 41 females (ewes, cows and goats) with mastitis. The isolation and purification of the strains was performed on blood agar at 37°C. Bacteria identification was done by conventional methods in quality control laboratory of the Tebessa Public Health Department. The selective medium used for gram+ bacteria (Staphylococcus and Streptococci) was the Chapman medium.
The catalase test was performed for the identification of staphylococci (Catalase positive: Staphylococci, Catalase negative: Streptococci). The coagulase test was used to identify S. aureus (coagulase positive) The sensitivity of the identified staphylococci to nine (9) antibiotics (Ampicillin (AM) 10μg, Oxacillin (OX) 1μg, Clindamycin (DA) 2μg, Vancomycin(VA) 30μg, Tetracycline (TE) 30μg, Erythromycin (E) 15μg, Cefotaxim (CTX) 30μg, Oflaxacin (OFX) 5μg) was studied using the standard antibiotic susceptibility method (dilution in agar medium). The discs used come from the Biomerieux laboratory (France). The results were interpreted according to the veterinary recommendations of the Antibiogram Committee of the French Society of Microbiology (AC-FSM VET, 2018).
Results and Discussion
In 41 milk samples, 84 bacterial strains were isolated, 73.81% were gram positive and 26.19% were gram negative (Table 1) Studies by Kadja et al. (2013) showed that 69.9% were gram positive bacteria and 30.10% were gram negative bacteria. The prevalence of staphylococcal mastitis was 61.91%, of which 57.15% were SCN, and 4.76% were CSP.
Table 1: Distribution of isolated bacteria according to their groups.
| Group of bacteria | Number | Percentage | ||
| Gram negatives | 22 | 26.19% | ||
|
Gram positive
|
Staphylococcus (61.91) |
CSP | 4 | 4.76% |
| CSN | 48 | 57.15% | ||
| Streptococci | 8 | 9.52% | ||
| Bacillus | 2 | 2.38% | ||
| Total | 84 | 100% | ||
Staphylococci have been the main cause of milk contamination during production (milking machine, infected cow, man, incomplete cleaning of the milking machine) (Thieulon, 2005).
In 2014, Smaali provided a prevalence of 72.4% for staphylococcal mastitis in sheep. On the other hand, Bergonier et al. (2005) found S. aureus in 1 to 8% of isolations in France, Spain and Sardinia. On the other hand, Beheshli and its collaborators (2010) contributed a rate of 88.40% for staphylococci isolated from milk.
The results of the antibiotic resistance profile study are reported in Figures 1, 2 and 3.
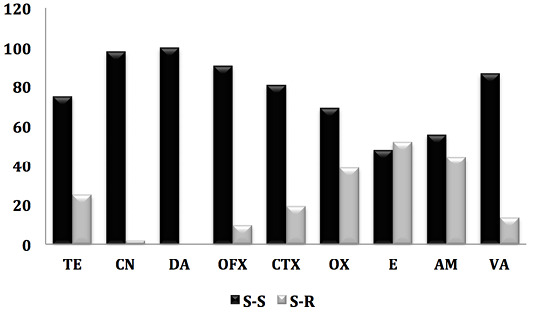
Figure 1: Antibiogram result of staphylococcus strains against 9 antibiotics. (S-S; e Staphylococcus-Sensitive, S-R; Staphylococcus-Resistant).
On all isolated (84 strains), the prevalence of methicilin-resistant staphylococci (oxicilin) was 19.45%, including 17.85% of CSN and 1.2% for MRSA. Indeed, work carried out in Saudi Arabia (Alzohairy, 2011) and Nigeria (Mai-siyama et al., 2014) reported prevalences of methecilin-resistant staphylococci by 15.5% and 28.8% respectively.
However, the detection of MRSA in sheep before slaughter once again confirms the risk to human health, not only for the consumer but also for all persons who come into contact with these animals (staff in establishments intended for the slaughter of animals, veterinarians, farmers) (Mai-siyama et al., 2014). Indeed, several studies have reported that close and repeated contact with his animals is a risk factor for the acquisition of MRSA-L. In addition, some of these strains may cause serious infections (Van Cleef et al., 2011). These at-risk individuals can then serve as reservoirs for MRSA-L and ensure its deamination in the community (Lekkerkerk et al., 2015).
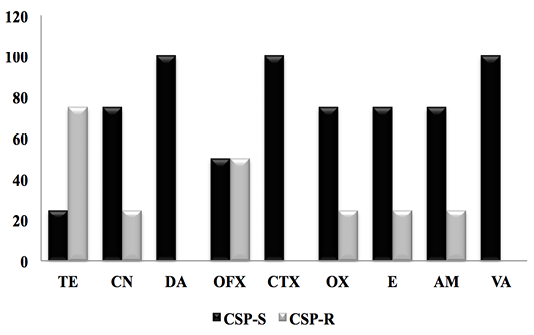
Figure 2: Antibiogram result of PCS strains against 9 antibiotics. (CSP-S; Coagulase Staphylococcus Positive-Sensitive, CSP-R; Coagulase Staphylococcus Positive-Resistant).
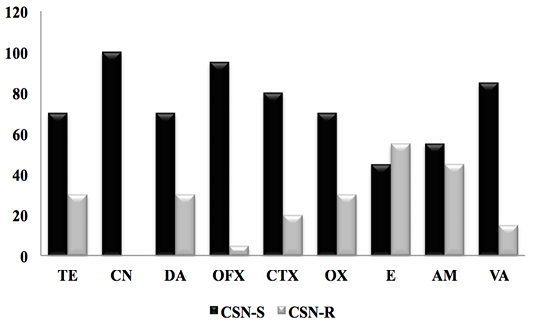
Figure 3: Result of the antibiotic susceptibility test of CSN strains against 9 antibiotics. (CSN-S; Coagulase Staphylococcus Negative-Sensitive, CSN-R; Coagulase Staphylococcus Negative-Resistant).
The results obtained (Figure 2) showed a very high resistance rate of CSP for tetracycline (75%), followed by ofloxacin (50%.), oxacillin, (25%.) erythromycin (25%) and ampicillin 25%. In contrast, CSP had 100% sensitivity to clindamycin, cefotaxime and vancomycin.
A recent study in the same region showed that SCPs were resistant to tetracycline (37.5%) and erytromicin (37.5%) and were sensitive to vancomycin (100%) (Smaali, 2019). Ben Hassen et al. (2003) showed that penicillin G resistance affected 64% of S. aureus strains, 36% for tetracycline and only 4% for oxacillin. For its part, Saidi (2014) provided a 100% resistance rate of S. aureus to Penicillin G.
In 2005, Turutoglu and al recorded a 61.1% resistance rate of S. aureus to Penicillin G in Turkey. Benhamed et al. (2011) isolated strains of S. aureus that were 100% methicillin sensitive.
In Malaysia, Jamali et al. (2014) obtained a resistance rate of 86% to penicillin and 76.6% to tetracycline.
The high percentage of resistance of S. aureus isolates to the b-lactamine family results from the acquisition of a B-lactamase gene and a plasmid penicillinase (Jamali et al., 2015). However, the resistance of these strains to Tetracycline is due to the extensive administration of this antibiotic on dairy farms (Jamali et al., 2015; Smaali, 2019).
For CSN strains, the antimicrobial resistance profile reported in Figure 3 showed a significant sensitivity of CSNs to clindamycin with a frequency (100%), followed by Oflaxacin (95%), vancomycin (85%) cefotaxime (80%), oxacillin and tetracycline with (70%), as well as ampicillin and erythromycin 55% and 45% respectively.
In 2003, Ben Hassen and al reported that CSNs have resistance to penicillin G with a percentage of (22.6%), tetracycline (20.8%), followed by streptomycin (16.5%) and erythromycin (16.5%), with low oxacillin resistance (1.7%).
Since their discovery, antibiotics have been used in veterinary medicine for curative purposes to treat sick animals, as a metaphysical treatment where animals in the same herd that are still healthy will receive the same treatment as sick animals and finally as a preventive or prophylactic measure to prevent certain diseases in animals identified as being at risk (Aarestrup, 2015). However, the misuse of antibiotics (insufficient doses, non-compliance with treatment duration) is most often at the root of this resistance.
The study of the antibiotic resistance profile of staphylococci (CSP and CSN) revealed the efficacy of vancomycin, clindamycin and oflaxacin against these pathogenic strains responsible for mastitis in runners.
Conclusion
The results of our study allowed us to conclude that the prevalence of methicillin-resistant staphylococcal mastitis was important (19.45%). The study of the resistance profile of the isolated strains shows the high resistance of these staphylococci to tetracycline. As well as the efficacy of vancomycin, clindamycin and oflaxacin against these pathogenic strains responsible for mastitis in runners. However, the presence of these multi resistant strains in ruminant livestock makes the antibiotic resistance problem in veterinary medicine even more complex than in human medicine.
To do this, Surveillance of these pathogenic bacteria in humans, animals and food allows us to identify the virulence factors of these pathogenic strains and establish appropriate control. However, the search for new, more effective molecules without side effects is necessary and we are moving towards the bioactive molecules of medicinal plants characteristic of Algeria, which will be the objective of our next articles.
In the meantime, it seems imperative to raise as much awareness as possible of the need to reduce the use of antibiotics as soon as possible, especially in veterinary medicine.
Acknowledgement
The authors express their deep gratitude to Mr. R. Brakni and all the quality control laboratory staff (public health department-Tebessi. Algeria).
Authors Contribution
The research was designed jointly by Smaali Saoussene,
Boukazoula Fatima, Saadi Lamia, Brahem Imen. Smaali
Saoussene conducted the research. The authors read and
approved the final manuscript.
Conflict of interest
The authors declare that there is no conflict of
interest.
References






