Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences. 2 (1): 26 – 30PMWS-like Lesions in Preweaned Crossbred Piglets Naturally Infected with Porcine Circovirus 2 in India
Rinku Sharma1, G. Saikumar2*
1. Regional Station, Indian Veterinary Research Institute, Palampur, Himachal Pradesh, India
2. National CSF Referral Laboratory and Swine Disease Laboratory, Division of Pathology, Indian Veterinary Research Institute, Izatnagar, Uttar Pradesh, India
*Corresponding author: [email protected]
ARTICLE CITATION: Sharma R and Saikumar G (2014). PMWS–like lesions in preweaned crossbred piglets naturally infected with porcine circovirus 2 in India. Adv. Anim. Vet. Sci. 2 (1): 26 – 30.
Received:2013–10–05, Revised:2013–11–10, Accepted: 2013–11–11
The electronic version of this article is the complete one and can be found online at (http://dx.doi.org/10.14737/journal.aavs/2014.2.1.26.30) which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Postweaning Multisystemic Wasting Syndrome (PMWS) caused by Porcine Circovirus2 (PCV2) is an economically devastating disease of pigs that generally affects pigs in the postweaning stage. In a pig breeding farm located in Northern India, where Landrace crossbred pigs are maintained, occurrence and pathology of PCV2 associated diseases was investigated. In all, 238 piglets from 70 litters farrowed by first parity gilts were screened for PCV2 infection by PCR. PCV2 infection was detected in 17 preweaned piglets representing 13 litters. Age–wise breakup of the PCV2 positive piglets revealed that 13 piglets were below 4 week of age and 4 piglets were aged 30, 37, 42 and 49 days old, respectively. Pathological lesions characteristic of PMWS were observed in 4 piglets aged 4–7 week old. PCV2 was demonstrated in lungs, heart, liver, kidneys, brain, lymph nodes, spleen and tonsil by immunohistochemistry and in situ hybridization in the tissues of the affected piglets. PCV2 positive pigs with PMWS–like lesions were negative for infection with Porcine Parvo Virus (PPV), Porcine Reproductive and Respiratory Syndrome Virus (PRRSV), Classical Swine Fever Virus (CSFV) and Aujeszky’s disease virus (ADV), as determined by PCR/RT–PCR. The results suggested that PCV2 infection was responsible for PMWS–like disease syndrome in preweaned piglets.
Introduction
Postweaning multisystemic wasting syndrome (PMWS) is a disease of pigs that is caused primarily by Porcine Circovirus 2 (PCV2) (Allan and Ellis, 2000). PMWS is commonly seen in 8–15 week old piglets and is characterized by clinical signs of wasting, reduced weight gain, jaundice, respiratory complications and lymphoid depletion (Ramamoorthy and Meng, 2008). PCV2 infection is ubiquitous in domestic pigs and PMWS has been diagnosed on all the five continents, including Australia where presence of viral infection has been demonstrated but the country is considered free of PMWS (Grau–Roma et al., 2011). Very often, additional factors besides the presence of PCV2 are necessary for the full expression of clinical disease (Tomas et al., 2008). The main factors attributed for the development of the clinical disease are co–infections with other pathogens (Krakowka et al., 2001; Rosell et al., 1999), immune system activation by vaccination (Krakowka et al., 2000; Kyriakis et al., 2002), host genetics (Lopez–Soria et al., 2005), management (Madec et al., 2008) and even the genotype of PCV2 involved (Tomas et al., 2008).
PMWS is now endemic in many swine–producing countries and continues to be a major cause of wasting disease in swine. Usually the disease has been reported in post weaned piglets, between 8–15 weeks of age, but here we describe the pathological changes and tissue distribution of PCV2 antigen and nucleic acid observed in preweaned piglets (below 8 weeks of age) naturally infected with PCV2 leading to development of PMWS lesions.
MATERIALS AND METHODS
Farm History
Reproductive problems and increased neonatal mortality was reported in a pig breeding unit located in North India where crossbred pigs (Landrace x local non–descript) were being reared. The farm practiced weaning after 8 weeks of birth and vaccination against classical swine fever (CSF), FMD and hemorrhagic septicemia was done regularly after weaning. The farm was a breeding unit which retained 40 gilts for subsequent breeding and disposed off all sows after first farrowing and weaned pigs at 10–12 weeks of age. The farm maintained 8–10 boars until culling was necessary on health grounds or when aged between 3–4 years. The study was conducted for two farrowings that occurred within a period of 12 months. The farm was positive for Porcine Parvovirus but negative for Porcine Reproductive and Respiratory Syndrome Virus, and Aujeszky’s disease virus.
Clinico–Epidemiological Observations
The general clinical observations in the affected litters included fetal mummification, low birth–weight, splay–leg condition, congenital tremors, and occasional presence of multiple, reddish–blue patchy areas of discoloration on the skin of thorax, abdomen and pelvic regions. A total of 594 piglets were farrowed by 70 sows with an average litter size of 8.65 piglets/litter. In 13 litters, the litter size was either 5 or less than 5 piglets with the lowest being 1 piglet in one litter. A total of 9 (1.5 %) mummified foetuses, 13 (2.2 %) stillborn and 572 (96.3 %) live born piglets were farrowed by 70 sows. Of the live born piglets, 238 (40 %) piglets died within 8 weeks of farrow. Out of these piglets, 194 (81.5 %) died within 7 days of age (neonates) and 44 (18.5 %) died within 8 weeks of age. The age distribution of 44 piglets was 8–14 days old (12), 15–21 days old (13), 22–28 days old (5), 29–35 days old (3), 36–42 days old (2), 43–49 days old (4) and 50–56 days old (5) piglets.
Gross Pathology and Histopathology
Detailed necropsy examination was conducted on dead piglets or those sacrificed in extremis. Gross pathological alterations were recorded and representative specimens from brain, tonsil, lungs, heart, liver, spleen, kidneys, stomach, intestine, and lymph nodes were collected on ice as well as in 10 % buffered neutral formalin. Tissue samples from lungs, liver, spleen and brain were also subjected to isolation studies. Formalin–fixed tissues were processed routinely to obtain paraffin–embedded and H & E stained sections for histopathological study (Luna, 1968).
Extraction of Genomic DNA and PCR Amplification
DNA extraction was carried out using the phenol–chloroform–isoamyl alcohol method from lungs, lymph nodes, spleen, liver, kidneys and brain tissues. Following DNA extraction, PCR targeting the ORF 2 gene of PCV2 was carried out using previously published primers which amplify a 264 bp product (Larochelle et al., 1999). The tissues were also screened by PCR/RT–PCR for the presence of Porcine Parvovirus (PPV) (Arnauld et al., 1998), Porcine Reproductive and Respiratory Syndrome virus (PRRSV) (Mardassi et al., 1994; Suarez et al., 1994), Aujeszky’s Disease virus (ADV) (Balasch et al., 1998) and Classical Swine Fever virus (CSFV) (Katz et al., 1993). Screening for the above viruses was based on the presence of clinical signs or suspected lesions at the time of post mortem, relating to that particular virus. For PPV, 61 animals from 31 litters were screened by PCR. For PRRSV, 25 serum samples from live animals and 67 animal tissues (collected at the time of post mortem), representing 44 litters were screened by PRRS X3, Herdchek kit, IDEXX Laboratories, Switzerland and RT–PCR. For PRV, 16 animals from 7 suspected litters were screened by PCR. For CSFV, 13 animals, from 10 litters were screened using RT–PCR test.
Immunohistochemical Detection of PCV2 Antigen
The representative paraffin–embedded tissue sections showing lesions were subjected to immunohistochemistry (IHC) for demonstration of PCV2 antigen using PCV2 specific polyclonal sera (VMRD Inc., USA) as described earlier (Kennedy et al., 2000).
In Situ Hybridization for Detection of PCV2 Nucleic Acid
Duplicate sections of paraffin–embedded tissues showing lesions were also subjected to in situ hybridization (ISH) for demonstration of PCV2 nucleic acid using previously described protocol (Choi and Chae, 1999) with certain modifications. Dig–11–dUTP labeled probe of 264 bp was generated by PCR using primers targeting the ORF 2 gene of PCV2 (Larochelle et al., 1999). Hybridization was carried out at 59oC for 10 hours in a slide hybridizer (Dako, Denmark).
RESULTS
PCR for PCV2 Detection
PCV2 nucleic acids could be detected in 13 of the 70 litters (18.6 %) tested and the number of PCV2 positive piglets was 17. In all, 13 piglets were below 4 weeks of age and 4 piglets were between 4 to 7 weeks of age. We present here the results of the 4 preweaned piglets between 4–7 weeks of age, which showed characteristic PMWS–like lesions. Out of these four piglets, 3 piglets aged, 30, 37 and 49 days old, respectively were from the same litter. The 30 days old piglet showed splay leg condition in neonatal stage. 7 live–born piglets were farrowed in this litter and an early neonatal mortality at 2 days was also received besides the above three. The fourth piglet under study was 42 days old. The litter size was 8 and 6 piglets were received for necropsy at 1 day, 2 days (2), 22 days, 30 days and 42 days of age. The PCR assay resulted in amplification of a 264 bp fragment from ORF2 gene of PCV2 genome. Viral nucleic acids could be detected in the lungs and lymph nodes. PPV, PRRSV, CSFV and ADV infection was not detected in the 2 litters.
Gross and Histopathological Lesions
The skeletal muscles appeared pale yellow and the carcass lymph nodes were enlarged and edematous. Lungs were non–collapsible, edematous, severely congested with patchy areas of consolidation in the diaphragmatic lobes. Heart was misshappened, flabby in consistency and pale yellow in color. Liver revealed mild enlargement and prominent yellowish–orange tan discoloration with patchy areas of congestion on the margins of lobes. Kidneys were enlarged and the thickened capsule peeled with difficulty to reveal a rough and uneven surface and multiple grayish–white patches or pale yellow discoloration of the organ.
Microscopically, lungs revealed mild to moderate interstitial pneumonia characterized by infiltration of lymphohistiocytic cells, proliferation of alveolar and interstitial macrophages, degeneration and desquamation of bronchiolar epithelial cells and alveolar haemorrhages. Heart revealed focal pericarditis, congestion, mild haemorrhages, edema, and vacuolar degeneration of epicardium, and degeneration of myocardiocytes with focal infiltration of mononuclear cells in the vicinity of congested blood vessels.
Lesions of hepatitis were characterized by widespread hepatocyte degeneration (fatty changes) and necrosis, multifocal infiltration of mononuclear cells, proliferation of Kupffer cells, disorganized hepatic cords, accumulation of lymphoid cells in the portal area, mild hyperplasia of bile duct epithelium, presence of necrotic cellular debris in lumen and increased connective tissue proliferation around bile ductules. Kidneys revealed marked congestion in the cortex and medulla, shrunken glomeruli, degenerative changes in the tubular epithelial cells of proximal and distal convoluted tubules. Lymph nodes and spleen showed depletion of lymphoid cells, marked proliferation of macrophages and occasional presence of syncytial cells which contained multiple variable–sized intracytoplasmic basophilic inclusion bodies. Brain revealed focal meningitis characterized by congestion and mononuclear cell infiltration. In the cerebral cortex, focal gliosis and infiltration of mononuclear cells around engorged blood vessels was also observed.
Immunohistochemical Detection of PCV2 Antigen
PCV2 antigen could be demonstrated mainly in the cytoplasm and occasionally in the nuclei of a wide range of lymphoid and non–lymphoid tissues such as lymph nodes, spleen, tonsil, lungs, heart, liver, kidneys and brain. In the follicular and interfollicular regions of the cortex and paracortex of lymph nodes, PCV2 antigen was detected in the macrophages and other reticuloendothelial cells containing multiple intracytoplasmic inclusions. In spleen, PCV2 antigen was observed in the mononuclear cells of depleted periarteriolar lymphoid sheath. In the tonsils, PCV2 antigen was detected in the degenerating crypt epithelial cells and macrophages in the follicular region and around the crypt. In lungs, PCV2 antigen was detected in the epithelial cells of alveoli, bronchi, bronchioles, and within the macrophages in the interstitium (Figure 1). In heart, antigen labelling was noticed in the myocardiocytes and infiltrating mononuclear cells. In liver, PCV2 antigen was observed in degenerating hepatocytes and mononuclear cells in the sinusoids. In the kidneys, PCV2 labelling was noticed mainly in the degenerating lining epithelial cells of tubules. Brain of one piglet revealed scanty presence of PCV2 antigen mainly in the lining endothelial cells of capillaries.
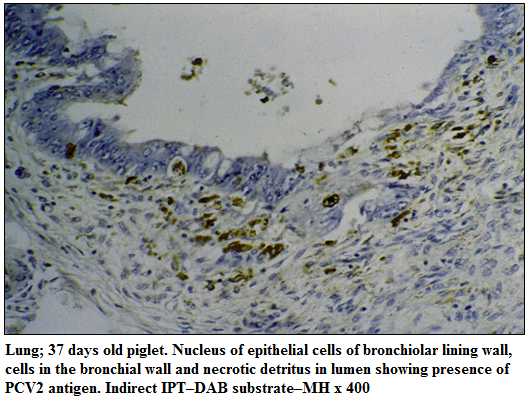
Figure 1:Lung; 37 days old piglet. Nucleus of epithelial cells of bronchiolar lining wall, cells in the bronchial wall and necrotic detritus in lumen showing presence of PCV2 antigen. Indirect IPT–DAB substrate–MH x 400
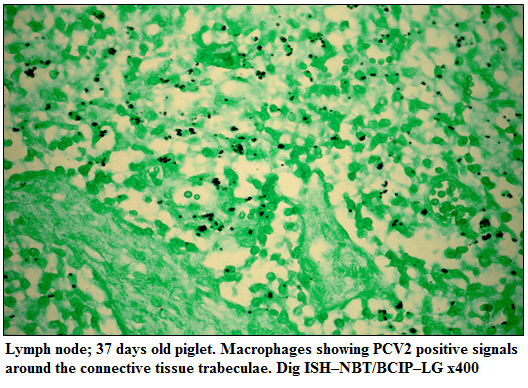
Figure 2:Lymph node; 37 days old piglet. Macrophages showing PCV2 positive signals around the connective tissue trabeculae. Dig ISH–NBT/BCIP–LG x400
In Situ Hybridization for Detection of PCV2 Nucleic Acid
The PCV2 nucleic acid was detected in lungs, heart, liver, kidneys, lymph nodes and spleen. The positive cells typically exhibited a dark brownish black reaction product mainly in the cytoplasm and occasionally in the nucleus of cells. In the lungs, hybridization signals were widely distributed in alveolar septal and interstitial macrophages and large mononuclear cells and cellular debris in alveolar lumen and bronchioles. Positive signals in heart were weak and noticed in the myocardiocytes. PCV2 nucleic acid was detected in the degenerating hepatocytes and in the tubular epithelial cells of kidneys. In the lymph nodes, multifocal hybridization signals were noticed in the macrophages and syncytial cells in the cortex, paracortex and medullary regions (Figure 2). Positive hybridization signals in the spleen were limited to large lymphoid cells in the periarteriolar lymphoid sheath and macrophages scattered in the white pulp
DISCUSSION
PMWS most commonly affects pigs from 2 to 4 months of age, the late–nursery and early–fattening phases of production (Grau–Roma et al., 2008). The results of the present study demonstrated that PCV2 was involved in the development of PMWS–like lesions in preweaned piglets aging less than 2 months old. Co–infecting viruses like PPV, PRRSV, ADV and CSFV were not detected in the affected litters. Pigs that are co–infected with other pathogens such as PRRSV, swine influenza virus (SIV) and Mycoplasma hyopneumoniae experience increased severity of Porcine Circovirus associated disease (PCVAD) (Dorr et al., 2007). However, it is not known whether the trigger for PCVAD manifestations in cases of co–infections with other pathogens arises from PCV2 or the co–infecting pathogens. Immunostress by other pathogens or even certain vaccines and adjuvants has an exacerbatory effect on PCV2 pathogenicity leading to development of PMWS–like lesions (Krakowka et al., 2000; Kyriakis et al., 2002). However, in India vaccination is generally done after 8 weeks of age. Therefore, probably factors other than vaccination and co–infections with PPV, PRRSV and ADV could be responsible for the PMWS–like disease observed in the present study. Further, the increased susceptibility of Landrace breed to development of PMWS (Lopez–Soria et al., 2005) may also have contributed to PMWS–like lesions in the piglets since this herd consisted of Landrace crosses. Previous investigations in this particular herd had shown reproductive failure due to PCV2 infection (Sharma and Saikumar, 2010), thus development of PMWS–like lesions in preweaned piglets might have occurred possibly due to in–utero infection which is known to occur in naturally infected gilts and sows (Ladekjaer–Mikkelsen et al., 2001; West et al., 1999). Also, the possibility of PCV2 alone leading to PMWS–like lesions in these piglets cannot be ruled out, which finds support from earlier studies reporting PCVAD due to PCV2 alone (Ellis et al., 2000; Grau–Roma et al., 2008; Opriessnig et al., 2006).
In the present study, pathological lesions in PCV2 infected piglets involved many organ systems and similar observation was made when pregnant sows were experimentally infected with PCV2 (Rovira et al., 2002). The clinical and gross findings were close to those observed in experimental or field cases of PMWS, except for the typical wasting of pigs (Krakowka et al., 2000). In the study, many piglets were weak and underweight, but the typical wasting of muscles which occurs gradually over a longer period of time was not noticed in these piglets which died before 60 days of birth. Our findings of interstitial pneumonia in preweaned piglets infected with PCV2 corroborated earlier observations made in experimentally induced PCV2 infection (Pallares et al., 2002). The heart lesions were less severe than those described previously (Mikami et al., 2005; O’Connor et al., 2001; West et al., 1999) however, our findings supported the earlier observations made by these authors that the cardiovascular system in general and endothelial cells in particular plays an important role in the pathogenesis of PCV2–associated diseases. The lesions in liver, kidneys, spleen and lymph nodes were similar to previous observations in weaner pigs diagnosed with PMWS (Perez–Martin et al., 2007; Rovira et al., 2002).
Presence and distribution of PCV2 antigen in various target tissues showing lesions characteristic of PMWS were similar to previous findings (Brunborg et al., 2007; O’Connor et al., 2001; Pallares et al., 2002; Rovira et al., 2002). Further, viral nucleic acid could be successfully demonstrated by ISH using DIG–labeled probe in lungs, heart, liver, lymph nodes and tonsil and these findings were similar to those reported earlier (Kim and Chae, 2001; Park et al., 2005). In the present study, a 30 day–old piglet with splay legs revealed PCV2 antigen labeling in the endothelial cells of capillaries and degenerating neurons. These findings agree with reported observations of brain lesions and PCV2 antigen distribution in PMWS affected pigs in Brazil (Correa et al., 2007). Since some conflicting observations on the direct association of PCV2 with congenital tremors and ataxia in piglets have been reported (Kennedy et al., 2003; Stevenson et al., 2001), this aspect needs more in–depth investigation. It is inferred from the study that natural PCV2 infection associated PMWS–like lesions were recorded in crossbred Landrace piglets in the preweaning stage in India. Detection of PCV2 antigen and nucleic acids by immunohistochemistry, PCR and in situ–hybridization techniques in lungs, heart, liver, kidneys, brain, lymph nodes, spleen and tonsil with lesions characteristic for PMWS indicated that early onset of the disease occurred but the commonly encountered co–infections in other countries were not found associated with these cases. Factors that led to early precipitation of PMWS–like disease need to be identified.
ACKNOWLEDGEMENT
The authors acknowledge the Director, IVRI, Izatnagar and ICAR, New Delhi for providing necessary facilities for conducting this investigation.
CONFLICT OF INTEREST
No conflict of interest to declare.
REFERENCES
Allan GM and Ellis JA (2000). Porcine circoviruses: a review. J. Vet. Diagn. Invest. 12: 3–14.
http://dx.doi.org/10.1177/104063870001200102
PMid:10690769
Arnauld C, Legeay O, Laurian Y, Thiery R, Denis M, Blandchard P. and Jestin A. (1998). Development of a PCR–based method coupled with a microplate colorimetric assay for the detection of PPV and application to diagnosis in piglet tissues and human plasma. Mol. Cell. Probes. 12: 407–416.
http://dx.doi.org/10.1006/mcpr.1998.0205
PMid:9843658
Balasch M, Pujols J, Segales J and Pumarola M (1998). Aujeszky's disease (PR) virus detection in cerebrospinal fluid in experimentally infected pigs. Vet. Microbiol. 60: 99–106.
http://dx.doi.org/10.1016/S0378-1135(97)00156-9
Brunborg IM, Jonassen CM, Moldal T, Bratberg B, Lium B, Koenen F and Schonheit J (2007). Association of Myocarditis with High Viral Load of Porcine Circovirus Type 2 in Several Tissues in Cases of Fetal Death and High Mortality in Piglets. A Case Study. J. Vet. Diagn. Invest. 19: 368–375.
http://dx.doi.org/10.1177/104063870701900405
PMid:17609345
Choi C and Chae C (1999). In situ hybridization for detection of PCV in pigs with PMWS. J. Comp. Pathol. 121: 265–270.
http://dx.doi.org/10.1053/jcpa.1999.0315
PMid:10486162
Correa AMR, Zlotowski P, Barcellos DESN, Cruz CEF and Driemeieri D (2007). Brain lesions in pigs affected with postweaning multisystemic wasting syndrome. J. Vet. Diagn. Invest. 19: 109–112.
http://dx.doi.org/10.1177/104063870701900120
PMid:17459844
Dorr PM, Baker RB, Almond GW, Wayne SR and Gebreyes WA (2007). Epidemiologic assessment of porcine circovirus type 2 co–infection with other pathogens in swine. J. American. Vet. Med. Assoc. 230: 244–250.
http://dx.doi.org/10.2460/javma.230.2.244
PMid:17223759
Ellis JA, Bratanich A, Clark EG, Allan G, Meehan B, Haines DM, Harding J, West KH, Krakowka S, Konoby C, Hassard L, Martin K and McNeilly F (2000). Co–infection by porcine circoviruses and porcine parvovirus in pigs with naturally acquired postweaning multisystemic wasting syndrome. J. Vet. Diagn. Invest. 12: 21–27.
http://dx.doi.org/10.1177/104063870001200104
PMid:10690771
Grau–Roma L, Crisci E, Sibila M, Lopez–Soria S, Nofrarias M, Cortey M, Fraile L, Olvera A and Segales J (2008). A proposal on porcine circovirus type 2 (PCV2) genotype definition and their relation with postweaning multisystemic wasting syndrome (PMWS) occurrence. Vet. Microbiol. 128: 23–35.
http://dx.doi.org/10.1016/j.vetmic.2007.09.007
PMid:17976930
Grau–Roma L, Fraile L and Segales J (2011). Recent advances in the epidemiology, diagnosis and control of diseases caused by porcine circovirus type 2. Vet. J. 187: 23–32.
http://dx.doi.org/10.1016/j.tvjl.2010.01.018
PMid:20211570
Katz JB, Ridpath JF and Bolin SR (1993). Presumptive diagnostic differentiation of hog cholera virus from BVD and BDV by using a cDNA nested–amplification approach. J. Clin. Microbiol. 3: 565–568.
Kennedy S, Moffett D, McNeilly F, Meehan B, Ellis J, Krakowka S and Allan GM (2000). Reproduction of lesions of PMWS by infection of conventional pigs with PCV alone or in combination with PPV. J. Comp. Pathol. 122: 9–24.
http://dx.doi.org/10.1053/jcpa.1999.0337
PMid:10627387
Kennedy S, Segales J, Rovira A, Scholes S, Domingo M, Moffett D, Mechan B, O'Neill R, McNeilly F and Allan G (2003). Absence of evidence of porcine circovirus infection in pigs with congenital tremors. J. Vet. Diagn. Invest. 15: 151–156.
http://dx.doi.org/10.1177/104063870301500209
PMid:12661725
Kim J and Chae C (2001). Differentiation of PCV l and 2 in formalin–fixed paraffin–wax–embedded tissues from pigs with PMWS by ISH. Res. Vet. Sci. 70: 265–269.
http://dx.doi.org/10.1053/rvsc.2001.0471
PMid:11676625
Krakowka S, Ellis JA, McNeilly F, Ringler S, Rings DM and Allan G (2001). Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with PCV2. Vet. Pathol. 38: 31–42.
http://dx.doi.org/10.1354/vp.38-1-31
PMid:11199162
Krakowka S, Ellis JA, Meehan B, Kennedy S, McNeilly F and Allan G (2000). Viral wasting syndrome of swine: experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by co–infection with porcine circovirus 2 and porcine parvovirus. Vet. Pathol. 37: 254–263.
http://dx.doi.org/10.1354/vp.37-3-254
PMid:10810990
Kyriakis SC, Saoulidis K, Lekka S, Miliotis CC, Papoutsis PA and Kennedy S (2002). The effects of immuno–modulation on the clinical and pathological expression of postweaning multisystemic wasting syndrome. J. Comp. Pathol. 126: 38–46.
http://dx.doi.org/10.1053/jcpa.2001.0520
PMid:11814320
Ladekjaer–Mikkelsen AS, Nielsen J, Storgaard T, Botner A, Allan G and McNeilly F (2001). Transplacental infection with PCV–2 associated with reproductive failure in a gilt. Vet. Rec. 148: 759–760.
PMid:11442241
Larochelle R, Antaya M, Morin M and Magar R (1999). Typing of PCV in clinical specimens by multiplex PCR. J. Virol. Methods 80: 69–75.
http://dx.doi.org/10.1016/S0166-0934(99)00032-4
Lopez–Soria S, Segales J, Rose N, Vinas MJ, Blanchard P, Madec F, Jestin A, Casal J and Domingo M (2005). An exploratory study on risk factors for postweaning multisystemic wasting syndrome (PMWS) in Spain. Prev. Vet. Med. 69: 97–107.
http://dx.doi.org/10.1016/j.prevetmed.2004.11.015
PMid:15899299
Luna LG 1968. Manual of histological staining methods of Armed Forces Institute of Pathology. 3rd edn Mc Graw Hill Book Company, New York, USA, pp 32–36.
Madec F, Rose N, Grasland B, Cariolet R and Jestin A (2008). Post–weaning multisystemic wasting syndrome and other PCV2–related problems in pigs: a 12–year experience. Transbound. Emerg. Dis. 55: 273– 283.
http://dx.doi.org/10.1111/j.1865-1682.2008.01035.x
PMid:18631230
Mardassi H, Wilson L, Mounir S and Dea S (1994). Detection of porcine reproductive and respiratory syndrome virus and efficient differentiation between Canadian and European strains by reverse transcription and PCR amplification. J. Clin. Microbiol. 32: 2197–2203.
PMid:7814546 PMCid:PMC263966
Mikami O, Nakajima H, Kawashima K, Yoshi M and Nakajima Y (2005). Nonsuppurative myocarditis caused by PCV type 2 in a weak–born piglet. J. Vet. Med. Sci. 67: 735–738.
http://dx.doi.org/10.1292/jvms.67.735
PMid:16082126
O'Connor B, Grauvreau H, West K, Bogdan J, Ayroud M, Clark EG, Konoby C, Allan G and Ellis JA (2001). Multiple PCV2–associated abortions and reproductive failure in a multisite swine production unit. Canadian Vet. J. 42: 551–553.
PMid:11467184 PMCid:PMC1476641
Opriessnig T, Fenaux M, Thomas P, Hoogland MJ, Rothschild MF, Meng XJ and Halbur PG (2006). Evidence of breed–dependent differences in susceptibility to PCV type– 2 associated diseases and lesions. Vet. Pathol. 43: 281–293.
http://dx.doi.org/10.1354/vp.43-3-281
PMid:16672575
Pallares FJ, Halbur PG, Opriessnig T, Sorden SD, Villar D, Janke BH, Yaeger MJ, Larson DJ, Schwartz KJ, Yoon KJ and Hoffman LJ (2002). Porcine circovirus type 2 (PCV–2) coinfections in US field cases of postweaning multisystemic wasting syndrome (PMWS). J. Vet. Diagn. Invest. 14: 515–519.
http://dx.doi.org/10.1177/104063870201400614
PMid:12423038
Park JS, Kim J, Ha Y, Jung K, Choi C, Lim JK, Kim SH and Chae C (2005). Birth abnormalities in pregnant sows infected intranasally with PCV–2. J. Comp. Pathol. 132: 139–144.
http://dx.doi.org/10.1016/j.jcpa.2004.09.003
PMid:15737340
Perez–Martin E, Rovira A, Calsamiglia M, Mankertz A, Rodrigues F and Segales J (2007). A new method to identify cell types that support PCV type 2 replication in formalin–fixed, paraffin–embedded swine tissues. J. Virol. Methods July 17; Epub ahead of print.
Ramamoorthy S and Meng X (2008). Porcine circoviruses: a minuscule yet mammoth paradox. Ani. Hlth. Res. Rev. 10: 1–20.
http://dx.doi.org/10.1017/S1466252308001461
PMid:18761774
Rosell C, Segales J, Plana–Duran J, Balasch M, Rodriguez–Arrioja GM, Kenneky S, Allan GM, McNeill F, Zatimer KS and Domingo M (1999). Pathological, immunohistochemical, and in situ hybridization studies of natural cases of PMWS in pigs. J. Comp. Pathol. 120: 59–78.
http://dx.doi.org/10.1053/jcpa.1998.0258
PMid:10098016
Rovira A, Balasch M, Segales J, Garcia L, Plana–Duran J, Rosell C, Ellerbrok H, Mankertz A and Domingo M (2002). Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J. Virol. 76: 3232–3239.
http://dx.doi.org/10.1128/JVI.76.7.3232-3239.2002
PMid:11884547 PMCid:PMC136035
Sanchez RE, Nauwynck HJ, McNeilly F, Allan GM and Pensaert MB (2001). PCV2 infection in swine fetuses inoculated at different stages of gestation. Vet. Microbiol. 83: 169–176.
http://dx.doi.org/10.1016/S0378-1135(01)00425-4
Sharma R and Saikumar G (2010). Porcine parvovirus– and porcine circovirus 2–associated reproductive failure and neonatal mortality in crossbred Indian pigs. Trop. Anim. Hlth. Prod. 42: 515–522.
http://dx.doi.org/10.1007/s11250-009-9454-0
PMid:19763866
Stevenson GW, Kiupel M, Mittal SK, Choi J, Latimer KS and Kanitz L (2001). Tissue distribution and genetic typing of porcine circoviruses in pigs with naturally occurring congenital tremors. J. Vet. Diagn. Invest. 13: 57–62.
http://dx.doi.org/10.1177/104063870101300111
PMid:11243364
Suarez P, Zardoya R, Prieto C, Solana A, Tabares E, Bautista JM and Castro JM (1994). Direct detection of the PRRSV by RT–PCR. Arch. Virol. 135: 89–99.
PMid:7545931
Tomas A, Fernandes LT, Valero O and Segales J (2008). A meta–analysis on experimental infections with porcine circovirus type 2 (PCV2). Vet. Microbiol. 132: 260–273.
http://dx.doi.org/10.1016/j.vetmic.2008.05.023
PMid:18614300
West KH, Bystrom JM, Wojnarowicz C, Shantz N, Jacobson M, Allan GM, Haines D, Clark EG, Krokowka S, McNeilly F, Konoby C, Martin K and Ellis JA (1999). Myocarditis and abortion associated with intrauterine infection of sows with PCV2. J. Vet. Diagn. Invest. 11: 530–553
http://dx.doi.org/10.1177/104063879901100608
PMid:12968736





