Advances in Animal and Veterinary Sciences
Research Article
Morphological Characteristics of the Goat Thyroid Glands among Summer and Winter Seasons
Sozan A. Ali*, Shafika A. El-Sayed, Nehal I.A. Goda, Rasha R. Beheiry
Department of Histology and Cytology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Egypt.
Abstract | Thyroid gland plays an important role where they produce thyroid and calcitonin hormones. The current investigation was performed on 20 healthy and freshly slaughtered adult male goats. The samples were collected then immediately fixed and processed for the histological and electron microscopical examination of thyroid gland in summer and winter seasons. Thyroid gland was surrounded by a connective capsule with fibrous septa dividing the parenchyma into lobules. Each lobule comprises of several thyroid follicles, also myoepithelial cells were surrounded these thyroid follicles. Semithin sections in the summer season showed that the parenchyma was formed from numerous numbers of inactive follicles, as opposed to the winter season where it had active thyroid follicles. Ultrastructural studies showed that in the summer season the thyroid follicles were lined with flattened to low cuboidal follicular cells, which had little cellular organelles. However, these follicles were lined with high columnar cells that engorged with the colloid droplets and other cell organelles in the winter season. Parafollicular cells were also found beneath the follicular cells that appeared elongated in shape with numerous darkly stained secretory granules.
Keywords | Thyroid gland, Follicle, Transmission electron microscope
Received | November 25, 2019; Accepted | February 17, 2020; Published | March 05, 2020
*Correspondence | Dr. Sozan A.A. Ismaeil, Assistant Prof. of Histology and Cytology, Faculty of Veterinary Medicine, Zagazig University, 44511 Zagazig, Egypt; Email: [email protected]
Citation | Ali SA, El-Sayed SA, Goda NIA, Beheiry RR (2020). Morphological characteristics of the goat thyroid glands among summer and winter seasons. Adv. Anim. Vet. Sci. 8(3): 252-259.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.3.252.259
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Ali et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The thyroid gland is the largest and the first recognizable gland during development in vertebrates (Dyce et al., 2017). In cattle, the gland is surrounded by a connective tissue capsule. This capsule comprises of two layers, the outer one consists of loose connective tissue while, the inner one consists of bundles of collagen and elastic fibers (Igbokwe and Ezeasor, 2015).
In donkey, the parenchyma consists of a number of lobules. Each lobule consists of several thyroid follicles. These follicles are in form of round, oval, tubular or polygonal in shape (Ali, 2014). In buffalo and pig, Hussin and Al-taay (2009) and Igbokwe and Ezeasor (2015) stated that the small-sized follicles are diffused at the periphery while the big follicles are found at the center.
In camels and african giant rat, Kausar and Shahid (2006) and Enemali et al. (2016) added that the small-sized follicles are lined by high cuboidal epithelium where they consider as active cells. In grass cutter and camel, the large-sized follicles have low cuboidal epithelium and even squamous epithelium in some very large follicles that are considered to be as an in-active cell (Igbokwe, 2010; Bello et al., 2015). Kausar and Shahid (2006) reported that the follicles are filled with a jelly-like colloidal material. The histochemical techniques revealed a positive PAS and negative Alcian blue reaction of colloid in broad-snouted caiman (Machado-santos et al., 2013).
Parafollicular cells (C cells) produce mainly calcitonin that controls calcium metabolism (Sawicki, 1995). Igbokwe and Ezeasor (2015) indicated that these cells are present in form of a single cell or in clusters. They are large oval or polyhedral in shape in grasscutter (Igbokwe, 2010). C-cells have large nuclei and lightly stained cytoplasm (Igbokwe and Ezeasor, 2017).
Atoji et al. (1999) noted countless rough endoplasmic reticulums in the cytoplasm of the follicular cells. However, smooth endoplasmic reticulums are less noticeable (Igbokwe et al., 2015). Dar et al. (2018) mentioned that the cytoplasm of these cells show the Golgi complex marked near the nucleus. The apical cytoplasm also indicates mild mitochondria figures and several colloid droplets. These cells apical boundary hold countless, and distinctly finger-like microvilli in goat (Igbokwe et al., 2015).
In bat, Pankaj et al. (2015) stated that few parafollicular cells present basally between two follicular cells close to the basement membrane. They occupy epifollicular position in hamster, while these cells occur in intrafollicular or interfollicular positions in rats (Hwang et al., 1986).
C-cells of bat are oval in shape with large oval nuclei and indented margin. They occupy a large portion of the cytoplasm (Pankaj et al., 2015). In goat, C-cells have an extensive mitochondrial profile and scanty rough endoplasmic reticulum. They have an amount of thick secretory granules of varying size and electron density. Lysosome-like bodies, autophagic vacuoles and residual bodies are also observed (Igbokwe et al., 2015).
The aim of this research work was to examine the histological and ultrastructural studies on thyroid gland of adult male goat by light and transmission electron microscope with special reference to the seasonal variations (summer and winter seasons). This work will provide interesting information which could have physiological application.
MATERIALS AND METHODS
Animals and tissue samples
The current study was conducted on 10 healthy, freshly slaughtered adult male goats “Capra hircus” of the same breed “Baladi breed” taken from Minia El-Kamh abattoir, Sharkia Governorate, Egypt during the summer season and the same number in the winter. Thyroid glands were dissected from the healthy animals immediately after slaughter. This work has been reviewed and approved by ZU-IACUC committee with approval number ZU-IACUC/2/F/39/ 2018.
Tissue preparation
The specimens were fixed in 10% buffered neutral formalin, then dehydrated followed by clearing in Xylol. All specimens were infiltrated with soft melted paraffin and were embedded in hard paraffin. Paraffin sections were stained with Harris’s Hematoxylin and Eosin (H and E) stain as a routine staining technique to show the general histological structure, Crossmon’s trichrome stain (for collagen and muscle fibers), Silver impregnation (for reticular fibers), Periodic Acid Schiff (PAS) (for neutral mucopolysaccharides and some acidic ones) and Alcian blue pH (2.5) (for acidic mucopolysaccharides) (Suvarna et al., 2018).
All the stained sections were examined with a standard light microscope (Olympus BX 21) and photographed by a digital Dsc-W 800 super steady cyper shot camera (Sony-Japan) at the Department of Histology and Cytology, Zagazig University).
Transmission electron microscopic (TEM) studies
Pieces of tissue about 1 mm³ were fixed immediately in buffered glutaraldehyde-formaldehyde fixative (GA/FA), then fixed in 1% osmium tetroxide and embedded in Epoxy resin. Semi-thin sections were cut on a MT2 Sorvall microtome and stained with toluidine blue. Ultrathin sections were cut on a RMC MT6000_ XL ultramicrotome, mounted on copper grids and stained with uranyl acetate and lead citrate. The ready ultrathin sections were examined and photographed at the Faculty of Agriculture, Mansoura University by a JEOL electron microscope (JEM 1200 EX II) operating at 80KV (Glauert and Lewis, 2014).
Histometrical analysis
Image J software (http://Sb.Info.nih.gov/ij/) is used for performing the quantitative measurements including; the diameter of large, medium and small-sized thyroid follicles and the height of the epithelium in both the active and inactive follicles in the winter and summer seasons.
In blood samples, serum was extracted from the different samples. The levels tri-iodothyronin and tetra-iodothyronin were measured in the different animals per each season.
Statistical analysis
All data were analyzed using SPSS version 24 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp). Results expressed as Mean ± SE. The independent samples t-test was used to compare differences between the previously measured parameters. Through which, P-value < 0.05 considered to be significant.
RESULTS
The thyroid gland was surrounded by connective tissue capsule, consisting of collagen and elastic fibers (Figure 1A). Connective tissue septa extended from this capsule dividing the parenchyma into lobules. These septa mainly had collagen fibers (Figure 1B). Each lobule formed from several thyroid follicles which might be small, medium or large in size. The small and medium-sized follicles could be seen at the periphery of the thyroid lobule. However, the larger follicles could be seen deeply (Figure 1C). The follicles of thyroid had distinct forms; an oval, round, elongated or irregular shape. These follicles were separated by interfollicular connective tissue which had little amount of collagen fibers, few elastic and reticular fibers with numerous blood capillaries (Figure 1D).
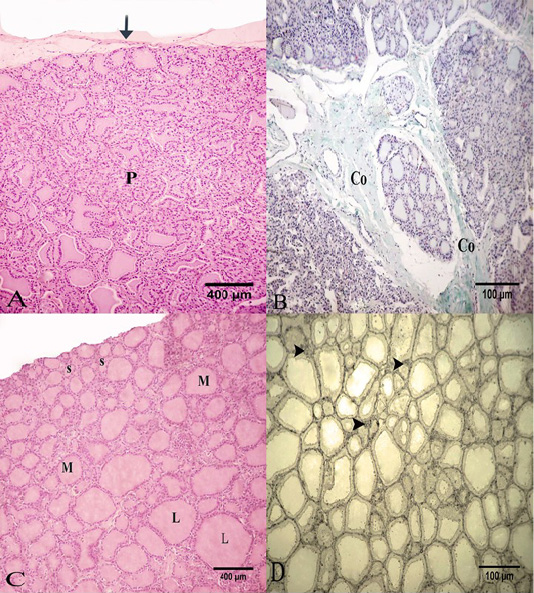
Figure 1: Photomicrograph of the goat’s thyroid gland showed that (A) Thyroid gland parenchyma “P” was surrounded by of a connective tissue capsule “arrow”. (B) Some connective tissue septa had mainly collagen fibers “Co”. (C) The parenchyma formed from several follicles; small “S”, medium “M” or large “L” in size. (D) The interfollicular connective tissue had little reticular fibers “arrow heads”. Stain: (A and C) H and E (B) Crossmon’strichrome (D) Silver impregnation (A and C) X.50, (B and D) X.100
In summer, most of the thyroid follicles were lined with simple squamous epithelium with flattened nuclei and the other follicles were lined with low cuboidal epithelium (Figure 2A). These follicles had homogenous and uniformly distributed colloid with a smooth profile. This colloid was positively reacted to PAS and Alcian Blue negative (Figure 2B). The semi-thin section of the thyroid gland in the summer showed that its parenchyma formed from a numerous number of inactive follicles. These follicles were lined with simple squamous epithelium and flattened nuclei. Some of these follicles were filled with luminal colloid with various densities. Some follicles had darker colloid and some had a paler one (Figure 3A).
In the summer season, ultrastructural characters showed that the thyroid follicles had flattened cells to low cuboidal cells with an electron-dense cytoplasm and heterochromatic nuclei, which occupy most area of the cells (Figure 3B). These cells had a moderate number of cell organelles including some colloid droplets, round lysosomes with an electron-dense contents and rough endoplasmic reticulum with dilated cisternae. These lysosomes were spread randomly within the cells (small and large) (Figure 4A and 4B).
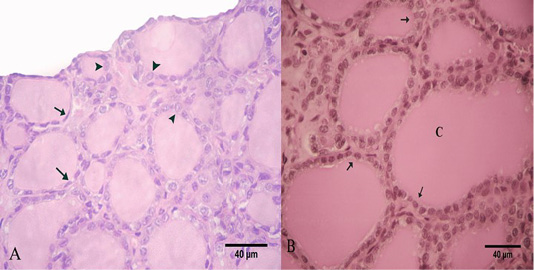
Figure 2: Photomicrograph of the goat’s thyroid gland showed that (A) In the summer season, most of the follicles were lined with simple squamous epithelium with flattened nuclei “arrows” and some follicles had low cuboidal epithelium “arrowheads”. (B) The follicles had a PAS-positive colloid “C” with smooth borders “arrows”. Stain: (A) H and E (B) PAS X.400.
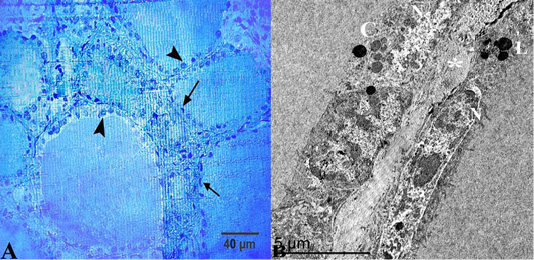
Figure 3: (A) Semithin section of thyroid gland in the summer season showed that thyroid follicles were lined with simple squamous epithelium with flattened nuclei “arrows” but some of them had low cuboidal epithelium “arrow heads”. (B) Ultrastructural characters showed that the thyroid follicles had flattened cells with an electron-dense cytoplasm and heterochromatic nuclei “N”, colloid droplets “C” and lysosomes “L”, these follicles were separated by interfollicular connective tissue “asterisk”. Stain:(A) Toluidine blue X.400.
In winter, most of the thyroid follicles were lined with high cuboidal epithelium with spherical nuclei. Most of these follicles have a highly vacuolated colloid (Figure 5A) that is positively stained by PAS (Figure 5B). Semithin section of the thyroid gland showed that the thyroid follicles had different shapes and sizes. These follicles lined with high cuboidal epithelium with parafollicular cells in between. They were surrounded by my oepithelial cells, which had flattened nuclei, and surrounded by blood capillaries (Figure 6A).
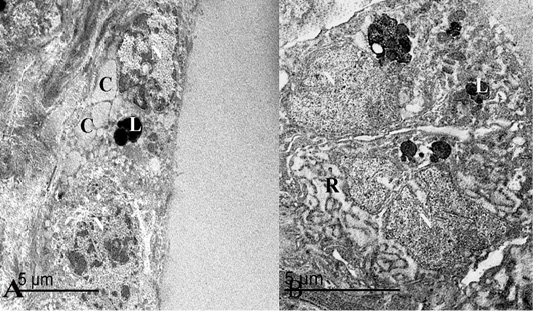
Figure 4: (A) An electron micrograph of thyroid gland in the summer season, showed that thyroid follicles were lined with flattened epithelium containing heterochromatic nuclei “N”, round lysosomes “L” and some colloid droplets “C”. (B) Follicular cells were low cuboidal in shape with heterochromatic nuclei “N”, lysosomes “L” and some rough endoplasmic reticulum “R”.
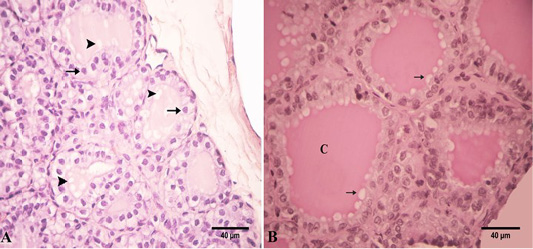
Figure 5: Photomicrograph of the goat’s thyroid gland showed that (A) In the winter season, thyroid follicles had high cuboidal epithelium with round nuclei “arrows”. These follicles had a vacuolated colloid “arrowheads”. (B) These follicles had a PAS-positive highly vacuolated “arrows” colloid “C”. Stain: (A) HandE (B) PAS X.400.
In the winter season, ultrastructural features of thyroid follicles showed that the follicular cells were high columnar in shape and nearly equal in length (Figure 6B). There are two kinds of follicular cells; active and inactive cells. The active type had two subtypes of cells. The first subtype is the during synthetic stage which had an electron-dense colloid droplets and the second subtype is the during secretory stage containing an electron-lucent colloid droplet. These follicular cells had ovoid heterochromatic compressed nuclei. Both subtypes had many colloid droplets, numerous mitochondria, and lysosomes. These colloid droplets compress on the adjacent nucleus displacing it to one side. These droplets were dispersed randomly within the cell (Figure 7A).
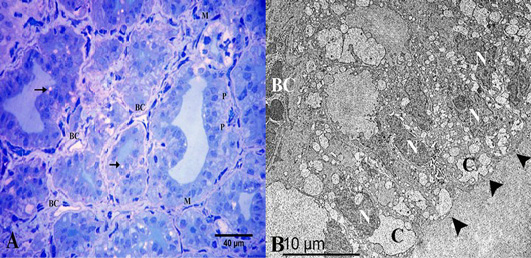
Figure 6: (A) Semithin section of thyroid gland in the winter season showed the thyroid follicles had high cuboidal epithelium, parafollicular cells in between “P” and vacuolated colloid “arrow”. They were surrounded by myoepithelial cells “M”. Note the thyroid follicles were surrounded by several blood capillaries “BC”. (B) Ultrastructural characters showed that the thyroid follicles had high columnar follicular cells “arrowheads” with heterochromatic nuclei “N” and several colloid droplets “C”. Note the blood capillary “BC”. Stain: (A) Toluidine blue X.400.
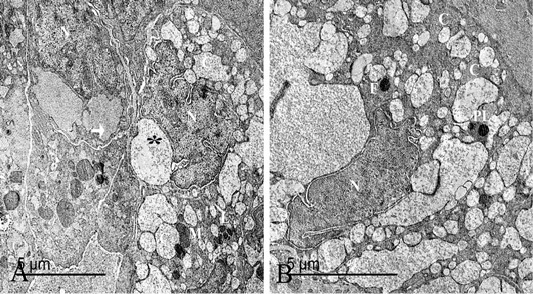
Figure 7: (A) An electron micrograph of thyroid gland in the winter season, showed synthetic subtype of the active follicular cells “arrow”, secretory subtype “star”, a compressed nucleus “N”, many colloid droplets “C”, numerous mitochondria “M” and lysosomes “L”. (B) The secretory follicular cell had a compressed nucleus “N”, many colloid droplets “C” and colloid filled endosomes fused “F” with the lysosomes forming phagolysosomes “PL”.
Colloid uptake occurred at the apical border of the follicular cells through pinocytosis. These colloid filled endosomes (electron-lucent) fused with the lysosomes (electron-dense) forming phagolysosomes. Phagolysosomes had areas of different densities resulting from the latter fusion. These droplets tended to be at the basolateral of the follicular cells (Figure 7B). The in-active follicular cells had an electron-dense cytoplasm with a few organelles.
Parafollicular cells were seen between the follicular epithelium as individual cells. They were found accidentally in thyroid sections. They were oval in shape, basally located and did not reach the lumen of the thyroid follicle. Parafollicular cells have oval nuclei and lightly stained cytoplasm. They could be seen clearly in the PAS-stained section. Additionally, thyroid follicles were surrounded by a thin sheet of myoepithelial cells. They were spindle-shaped cells with flattened nuclei (Figure 8).
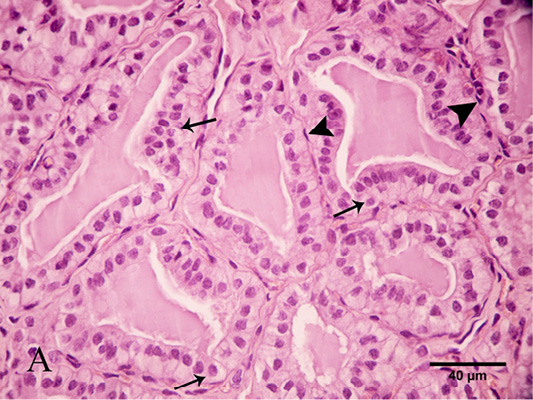
Figure 8: Photomicrograph of thyroid gland showed that parafollicular cells were seen between the follicular cells “arrows”. Note the thyroid follicles were surrounded by myoepithelial cells with flattened nuclei “arrow heads”. Stain: H and E X.400
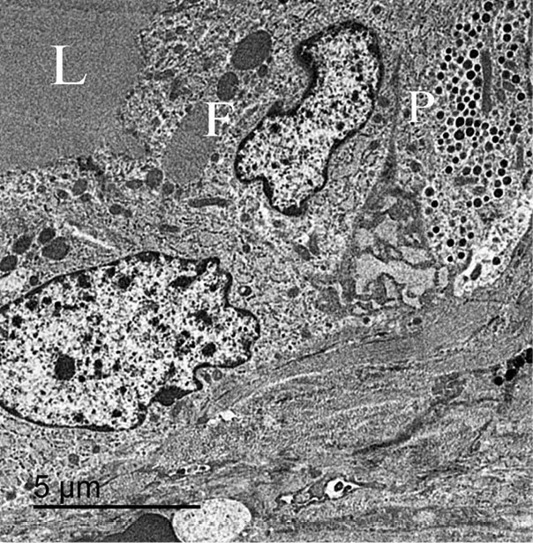
Figure 9: An electron micrograph of thyroid gland, showed that the parafollicular cells “P” present below follicular cells “F” and not reach the follicular lumen “L”.
Few parafollicular cells were accidentally encountered in sections. They were basally located to the follicular cells and never come in contact with the lumen (Figure 9). These cells had large and central nuclei. Its cytoplasm had many secretory granules and several elongated mitochondria. Their secretory granules were basally located, equal in size with electron dense homogenous contents (Figure 10).
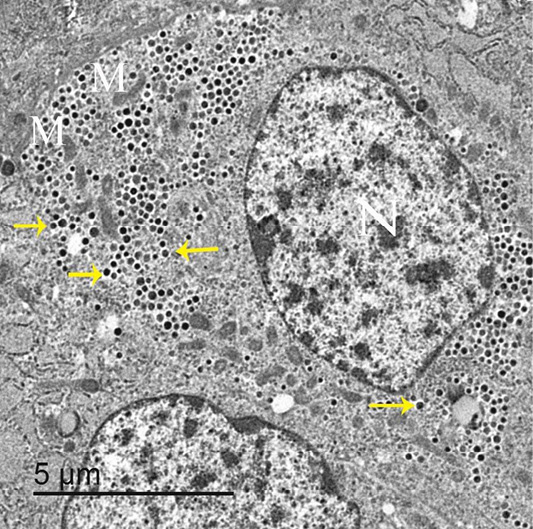
Figure 10: An electron micrograph of thyroid gland, showed that parafollicular cells had large and central nuclei “N”, elongated mitochondria “M” and darkly stained granules “arrows”.
Results of independent samples t-test revealed that the diameter of the small-sized follicles significantly differs in the summer and winter seasons mean ± SE = (23.05 ± 1.32 and 33.14±1.43) for the summer and the winter respectively. P < 0.001. However, there was a non-significant difference between the diameters of large-sized, a diameter of the medium sized follicle, the height of the epithelium of the active follicles, the height of the epithelium of the inactive follicles, T3 level and T4 level during the summer and winter seasons (P > 0.05) (Table 1).
DISCUSSION
The thyroid gland secretes thyroid hormones, including the hormone of tri-iodothyronine, tetra-iodothyronine and calcitonin. It considers as ductless organ where their hormones are released directly into the blood. These glands regulate body metabolism in different animals. The microscopic structure of the thyroid gland of the adult goats was similar to other domestic animals. It was surrounded by a connective tissue capsule. Similar results were reported by Hamad (2008) in goats.
In donkey, the parenchyma of the thyroid gland consisted of multiple numbers of lobules. Each lobule formed from
Table 1: Mean and standard error of mean of the follicular diameter, height of epithelium of the active and the inactive follicles of the thyroid gland and the levels of T3 and T4 in both the summer and the winter seasons.
| Parameter | Mean ± SE | Significant level | |
| Summer season | Winter season | ||
| Diameter of large sized follicles | 158.1 ± 3.86 | 155.4±2.51 | > 0.05 |
| Diameter of medium sized follicles | 64.02 ± 3.42 | 70.9 ±3.39 | > 0.05 |
| Diameter of small sized follicles | 23.05± 1.32 | 33.14 ±1.43 | < 0.001 |
| Height of the epithelium of the active follicles | 7.72 ± 0.44 | 7.53 ± 0.35 | > 0.05 |
| Height of the epithelium of the inactive follicles | 5.83 ± 0.44 | 5.36 ± 0.32 | > 0.05 |
| Level of the T3 hormone | 0.37 ± 0.04 | 0.36 ± 0.037 | > 0.05 |
| Level of the T4 hormone | 1.86 ± 0.146 | 1.79 ± 0.132 | > 0.05 |
numerous number of thyroid follicles (Ali, 2014). In the recent study, each lobule consists of part of the connective tissue capsule and several thyroid follicles. These follicles might be small, medium or large in size.
In our work, the small and medium-sized follicles were seen at the periphery of the thyroid lobe. However, the larger follicles were seen deeply. Similar findings have been reported in buffalo by Hussin and Al-taay (2009). In contrary to, Kausar and Shahid (2006) and Igbokwe and Ezeasor (2015) who mentioned that large and medium-sized follicles were found near the capsule in one-humped camel and pigs. These follicles may be irregular in form due to compression by other larger follicles (Machado-santos et al., 2013).
The follicles were filled with acidophilic colloidal material. The colloid is the storage form of the secretion of the follicular cells. The histochemical techniques revealed a positive PAS and negative AB reaction of colloid. It indicates the presence of a glycoprotein rich in neutral glycosaminoglycans. Similar results were ensured by the last author (Machado-santos et al., 2013).
In summer, the follicles were lined with low cuboidal epithelium or simple squamous epithelium. These follicles had homogenous and evenly distributed colloid with an outer smooth profile which indicates inactive thyroid follicles.
In the current research, the ultrastructural studies showed that thyroid follicles in the summer were lined with flattened cells to low cuboidal cells with an electron-dense cytoplasm and heterochromatic nuclei, which occupy most of the area of the cells. These cells had a moderate number of cell organelles including some colloid droplets, round lysosomes, and rough endoplasmic reticulum. Colloid droplets were little in number and small in size indicating lowering in thyroglobulin synthesis during the summer season (Igbokwe and Ezeasor, 2015). The apical borders of the follicular cells had short stunted microvilli, indicating minimal endocytotic activity. Fujita (1975) also added that lysosomes were also uncommon which ensures that the process of releasing thyroid hormones from thyroglobulin can be tedious.
Thyroid follicles were lined with high cuboidal epithelium in the winter season with a highly vacuolated colloid. The variation in the shape and size of the follicular cells might be due to climatic factors and different environmental conditions (Uchiyama et al., 1986).
In the winter season, the ultrastructural studies showed that follicular cells were columnar in shape. The follicular cells had two cases; active and inactive cells. The active follicular cell had heterochromatic compressed nuclei. They had countless colloid droplets of different patterns and sizes, numerous mitochondria and lysosomes. Abdel-Magied et al. (2000) reported that the existence of Golgi complexes, RER, and secretory vesicles suggests the activity of follicular cells in the synthesis and secretion of thyroglobulin towards the lumen. Kameda et al. (1986) added that the secretory colloid droplets, containing thyroglobulin were produced in the Golgi complex and transferred to the apical plasma membrane, where they release their contents by exocytosis into the follicular lumen.
Abdel-Magied et al. (2000) mentioned that lysosome- like bodies were seen in the cytoplasm of these cells. In the present work, colloid uptake happened in the apical portion of the follicular cells through endocytotic activity. These colloid filled endosomes fused with the lysosomes forming phagolysosomes. The fusion of colloid droplets and lysosomes ensures the functional role of the lysosomal enzyme in the release of thyroid hormones from thyroglobulin. Thus, thyroid hormones formed on the thyroglobulin scaffold could be processed intra-cellularly and released into circulation (Dar et al., 2018).
During low environmental temperature, pituitary thyroid-stimulating hormone was secreted which stimulates the thyroid cell growth, the synthesis, and secretion of thyroid hormones. TSH increases 1) iodide binding, 2) synthesis of iodotyrosines, 3) secretion of thyroglobulin into colloid and 4) endocytosis of colloid, blood flow, hypertrophy of the cells and weight of gland (Khatawkar and Awati, 2015).
In the recent study, few parafollicular cells were encountered in all follicles. They were found accidentally in thyroid sections. These cells were not present in the thyroid gland of the camel (Bello et al., 2015). These cells were reported in cattle (Igbokwe and Ezeasor, 2015). They were polyhedral or oval in shape, basally located and not reach the lumen of the thyroid follicle. C-cells have oval nuclei and lightly stained cytoplasm. They could be seen clearly in the PAS-stained section.
The current study showed that a thin sheet of myoepithelial cells surrounded the follicles. Similar results were mentioned by Hussin and Al-taay (2009) in buffalo. These cells assist to contract thyroid follicles and release their secretory products.
In rats, parafollicular cells are present in intra-follicular or inter-follicular locations (Hwang et al.,1986). The recent work showed that few parafollicular cells were encountered in sections. Parafollicular cells were elongated cells with oval or elongated nuclei. Its cytoplasm had numerous secretory granules, scanty rough endoplasmic reticulum, little Golgi apparatus, and many lysosomes. Its secretory granules were mainly basally located. Similar results were noted by Igbokwe et al. (2015).
The thyroid gland had follicles of different sizes in the summer and winter seasons. Most of the follicles were large in the summer season and small ones in the winter season (Abdel-Magied et al., 2000). In the recent study, the mean diameter of the small-sized follicles significantly differs in the summer and the winter season 23.05 ± 1.32 and 33.14±1.43 respectively. However, the mean diameter of the large-sized and the medium-sized follicles were 158.1 ± 3.86 μm and155.4±2.51 μm and 64.02 ± 3.42 μm and70.9 ±3.39 μm in the summer and the winter seasons respectively. The mean diameter of the small round follicles is 96.001 ± 0.03 μm. The medium follicles measured 180.18 ± 0.07 μm while the large round follicles are 240.01 ± 0.08 μm (Igbokwe, 2010).
The height of the epithelium of the active follicles (small) were 7.72 ± 0.44 μm and 7.53 ± 0.35 μm in the summer and the winter season respectively. However, the height of the epithelium of the inactive follicles (large) were 5.83 ± 0.44 μm and 5.36 ± 0.32 μm in the summer and the winter seasons respectively. The epithelial cell heights of the small, medium and large-sized follicles were 7.52 ± 0.05 μm, 7.31 ± 0.01 μm and 7.12 ± 0.06 μm respectively (Igbokwe, 2010). The diameter of the follicular cells in both seasons showed no significant difference (Hussin and Al-taay, 2009).
We found that the thyroid follicular cells were more active during the winter season with large and numerous colloid droplets which compress on the different cellular organelles. These organelles become less demarcated. While, during the summer season these follicular cells had small and few colloid droplets.
The current work revealed that the levels of T3 and T4 were clearly increased during the winter season which is related to the marked increase in the height of the follicular epithelium at the previously mentioned season. We also noticed that the parafollicular cells were found similarly in both seasons. Thus, it is not affected by the environmental temperature.
CONCLUSION
The thyroid gland consisted of several thyroid follicles. These follicles consisted of two cell types including, follicular and parafollicular cells. The thyroid follicles were surrounded by myoepithelial cells. The follicular cells were regarded to be inactive cells during the summer season and active ones during the winter season. During the winter season, they had numerous organelles and few organelles during the summer season.
ACKNOWLEDGEMENTS
This work was supported by Faculty of Veterinary Medicine, Zagazig University, Egypt.
Authors Contribution
Sozan A. Ali: Revision of the whole article, Improving the resolution of the images and shared as a corresponding author with the journal. Shafika A. El-Sayed: Editing the photos, repairing and revision of the whole article. Nehal I.A. Goda: Collection of the samples, application of the practical study, taking the photos of the light and electron microscope and scientific writing of the article. Rasha R. Beheiry: Revision of the article, examining the English language and improving the images quality.
Conflict of interest
There is no conflict of interest.
REFERENCES






