Advances in Animal and Veterinary Sciences
Research Article
Comparative Morphological and Structural Study of the Olfactory Mucosa among Adult Dog (Canis familiaris) and Goat (Capra hircus)
Sally A.M. Mohamed1*, Lamiaa L.M. Ebraheim1, Hoda F.A. Salem1, Shafika A. El-Sayed 1, Eman A. Ismail2
1Department of Histology and Cytology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44519, Egypt; 2Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Egypt.
Abstract | Olfaction is the process of odor detection that is important for many vital processes including determination of pheromones, food and hazard. The degree of olfaction showed great variation among herbivores and carnivores with highest degree in the later. The olfactory mucosa plays an important role in the development of such function, however, its morphological and structural differences among herbivores and carnivores is not fully understood. Therefore, in this study thirteen heads were obtained from both dog and goat and compared anatomically, histologically based on immunohistochemical staining for both glial fibrillary acidic protein``GFAP`` and synaptophysin and ultrastructurally among both species. Anatomically, the dog showed highly folded ethmoidal complex than that in the goat. Histologically, olfactory nerve cell density was statistically higher in the dog (174.7±2.7) compare to goat (56.3±2.2). In addition, the dog contained both the horizontal and globose basal cells where the goat had only globose one. Immunohistochemically, the area percentage of GFAP positive expressions in the olfactory mucosa was significantly increased in the dog than the goat. Ultrastructurally, the cilia emanate from around the base and the tip of the olfactory knob in the dog and goat, respectively. Olfactory ensheathing cells surrounded the nerve axons with space which narrow in the dog and wide in the goat. In conclusion, our results suggest that the olfactory mucosa of the dog is better specialized than that of the goat indicating a higher level of dependence on this system to catch their prey. Reciprocally, the goat has a relatively low olfactory sense as olfaction has primarily been confined to reproductive events.
Keywords | Ethmoturbinates, Olfactory receptor cells, Bowman’s gland, Immunohistochemistry, Ultrastructurally
Received | September 19, 2019; Accepted | October 26, 2019; Published | December 12, 2019
*Correspondence | Sally Abdelhamid Mohamed, Department of Histology and Cytology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44519, Egypt; Email: [email protected]
Citation | Mohamed SAM, Ebraheim LLM, Salem HFA, El-Sayed SA, Ismail EA (2019). Comparative morphological and structural study of the olfactory mucosa among adult dog (Canis familiaris) and goat (Capra hircus). Adv. Anim. Vet. Sci. 7(s2): 175-182.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s2.175.182
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Mohamed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Carnivores especially dogs have a well-developed sense of smell by which they could discover very tiny concentrations of odoriferous substances (Laska, 2017). Furthermore, dogs depend on the olfactory sense for hunting, herding, pulling loads, protection, assisting police and military as chemical detectors for explosives (Furton and Myers, 2001). On the other hand, herbivores such as the goat, showed a limited role of olfaction where they depend on it during reproductive events such as mating, estrus and mother–infant interaction (Gelez and Fabre-Nys, 2004). However, little has been reported concerning the morphological and structural difference among both species. Therefore, the current study focuses on comparing the structural features of the olfactory mucosa in both dog and goat that are predictive of the differences in olfactory capabilities in comparison to the feeding behaviors of the two species.
The olfactory mucosa of mammals is positioned in the dorso-caudal part of the nasal cavity where it mainly lines the ethmoturbinates (Onyono et al., 2017). Architecturally, the olfactory mucosa is composed of epithelium and an underlying lamina propria (Kavoi et al., 2010). The epithelium contains olfactory receptor neurons, supporting and basal cells (Barrios et al., 2014). Olfactory receptor neurons are responsible for sensory transduction and signaling that project a single dendrite with the olfactory knob that extends to the epithelial surface and holds nonmotile, sensory cilia where odor molecules bind to their receptor and an axon that transmits signals to the brain (Anholt, 1993). Axons from these olfactory neurons form nerve bundles (fila olfactoria) that cross the cribriform plate to synapse with other neurons in the glomeruli of the olfactory bulb (Pinto, 2011). Olfactory receptor neurons continuously produced synaptophysin and transport this protein to their terminals in the olfactory glomeruli (Bergmann et al., 1993) and detected by using anti-synaptophysin antibodies. Also, glial processes of olfactory ensheathing cells that encircle olfactory neurons detected by anti-GFAB antibodies (Crespo et al., 2019). We hypothesize that the variations of the olfactory mucosa structure correlate with the olfactory capabilities in both studied species and consequently their ecological behaviors. Therefore, our study was focus on differences in the olfactory mucosa of both dogs (carnivore) and goats (herbivore), based on anatomical, histological, immunohistochemical, and ultrastructural analyses.
Material and Methods
Animals and ethical considerations
Thirteen male Balady dogs (Canis familiaris), aged approximately 1-1.5 years and another thirteen male goats (Capra hircus), aged approximately 1-1.5 years were obtained from the laboratory animals research Center, Faculty of Veterinary Medicine, Zagazig University, Egypt. Dogs were housed in a hygienic kennel that was well ventilated with a temperature of 18 °C and relative humidity of 65 %. Dogs, food composed of fresh cocked minced cow meat and boiled rice provided in a clean bowl. Goats were kept on free-range pastures, mainly shrubs. At night, they were sheltered in the animal house kept at 22-25 °C with a humidity of 60% with a floor bedding of sawdust. The water was provided ad libitum for both species. Both species were kept under observation for four weeks before the commencement of tissue harvesting. The protocol in this research has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), Zagazig University (ZUIACUC/ 2/F/57/2018).
Tissues collection
The dogs were tranquilized by intramuscular injection of xylazine (0.5 mg/kg). The animals were then anesthetized by i.v. injection of 20-25 mg/kg thiopental (Hall et al., 2001). After that, the animals were subjected to a common carotid arteries perfusion with cold 4% paraformaldehyde (PFA) in 0.1M PBS for light microscopy and immunohistochemistry and 4% PFA+ 2.5% glutaraldehyde for complete examinations under TEM. After the euthanasia of all animals, the lower jaws were separated and para-sagittal section was made in one head from both species to reveal the ethmoturbinates. Another one frozen head of both species was sectioned into a transverse plane at the level of lateral canthi of both eyes.
Tissue preparation for light microscopy examination
Dissected the ethmoidal conchae of paraformaldehyde perfused fixed heads from 6 dogs and another 6 goats were immediately immersed with 4% paraformaldehyde in PBS overnight at 4 °C then processed till obtaining paraffin sections. All specimens were processed and followed by staining with HandE for histological analysis (Suvarna et al., 2019). The other deparaffinized sections were utilized for immunohistochemical analysis (Noreldin et al., 2016; Noreldin et al., 2018). Then, these sections were hydrated, washed in PBS at pH 7.2 (5 minutes). Such sections were immersed in absolute methanol containing 3% H2O2 to block endogenous peroxidase activity for 30 min at 4 °C. Followed by rinsing with water and incubation with 10% normal goat serum (blocking reagent) at room temperature for 1h to reduce unspecific binding of immunoglobulins. Then sections were incubated overnight at 4 °C with the primary antibody dilutions, rabbit polyclonal anti-GFAP antibody (Glial Fibrillary Acidic Protein) (Cat. No. RB-087-R7, Lab Vision, Thermo Scientific, Thermo Fisher Scientific), rabbit monoclonal anti-Synaptophysin (Neuroendocrine marker), (Cat No. RM-9111-PCL, Lab Vision, Thermo Scientific, Thermo Fisher Scientific), rabbit monoclonal anti-Chromogranin A (Cat. No. RM-9112-R7, Lab Vision, Thermo Scientific, Thermo Fisher Scientific). The specificity of primary antibodies for dog and goat tissues was validated by using normal rabbit IgG on dog and goat nervous tissues by the same primary antibodies, concentrations to represent as a negative control. The primary antibody was then incubated at 4°C overnight. After washing with PBS, the sections were incubated with biotin-conjugated goat anti-rabbit IgG antiserum (Histofine kit, Nichirei Corporation, Japan) for 60 min. Then washed in PBS, followed by incubation with streptavidin-peroxidase conjugate (Histofine kit, Nichirei Corporation, Japan) for 30 min. The streptavidin-biotin complex was visualized with 3, 3′-diaminobenzidine tetrahydrochloride (DAB)-H2O2 solution, pH 7.0, for 3 min. Then sections were washed in distilled water and Mayer’s hematoxylin was used as a counterstain. Photomicrographs from the stained sections were obtained with a digital camera (Leica EC3, Leica, Germany) connected to a microscope (Leica DM500 Germany).
Tissue preparation for transmission electron microscopy examination
Dissected Glutaraldehyde-fixed olfactory mucosa of Glutaraldehyde perfused fixed heads from one dog and another one goat were post-fixed with 1% osmium tetroxide in 0.1 mol/L phosphate buffer for 2 hours. Specimens were then dehydrated with an ascending series of alcohol concentrations and embedded in epoxy resin. These resin blocks were cut at a thickness of 75 nm, stained with uranyl acetate and lead citrate (Chevillas and Stasko, 2014) and then examined with transmission electron microscopy using a JEOL-JEM-2100 electron microscope at the Electron Microscopic Unit, Faculty of Agriculture, Mansoura University, Egypt.
Morphometric analysis
For the morphometric study, six animals were utilized per species. For measuring the mucosal thickness (epithelium and lamina propria), we used the Image J analysis software (Fiji image j; 1.51 n, NIH, USA) and representative fields scattered in the captured photomicrographs at 100× magnification that were selected depends on the presence of well-defined ethmoturbinate at longitudinal sections. The number of olfactory receptor neurons (ORNs) in the outer epithelial surface from the ethmoidal conchae was also counted per standard field area (5917.4µm) at 400× magnification. In addition, the area percentage of GFAP and synaptophysin immuno-positive expressions were also measured in the captured representative immunohistochemical fields at 40× magnification according to (El-behery et al., 2019). Briefly, we used immunohistochemical images where in the brown areas are positive reactions stained by DAB. The color deconvolution was then applied to these images via H DAB matrices of an image J software. On DAB matrices, the images were changed to grayscale image (Type–8bit) then adjust the threshold to detect only the DAB positive according to intensity. The threshold parameter was constantly throughout analysis for all immunohistochemical images. The average area percentages for both species were recorded.
Statistical analysis
The obtained numerical values from both dogs and goats were analyzed via using Independent student’s t in SPSS software program (version 16.0; Chicago, USA). Data were reported as means ± standard error (SE) and statistical significance determined at p ≤ 0.05, n=6/ species.
Results and Discussion
Anatomical features of the turbinate complex in the nasal cavities
The nasal cavities form bilaterally symmetric compartments by the nasal septum. The nasal conchae (conchae nasals) were ossified scrolls like structure project into the interior of the nasal cavity which covered with nasal mucosa (respiratory and olfactory). They are dorsal, ventral, middle and ethmoidal conchae (Figure 1A, B). The caudal part of dorsal, middle and ethmoidal conchae covered with olfactory mucosa. It characterized by dark brown color in the dog and yellow color in the goat unlike, the rostral respiratory mucosa which had a bright-red color (Figure 1A, B). There was bony shelf (lamina transversalis posterior) which separated the ventral nasopharynx from the dorsal ethmoidal conchae. Transverse sections of the heads show more clearly complex turbinates in the dog than the goat. In the dog, the ethmoidal conchae extended more rostrally and its scrolls were more folded in contrast to the goat where the turbinates had a short and clear cylindrical scroll without folds. The ethmoidal conchae were arranged in two sets; one interior (Endoturbinates) and another exterior (Ectoturbinates) which numbered 4 and 6 in the dog and 5-6 and 9-10 in the goat, respectively (Figure 1C, D).
Histological features of the olfactory mucosa
The olfactory mucosa was observed to be consisted of outer and inner surface epithelium, and lamina propria in between that consisted of connective tissue harboring Bowman`s glands beneath olfactory epithelium, olfactory nerve axon bundles, and bone (the bony scrolls of the ethmoidal conchae) (Figure 2A, B).
Interestingly, in dog both of the outer and inner epithelial surfaces were of olfactory type. However, in goat only the outer epithelial lining was of olfactory type, but the inner surface was lined by respiratory epithelium (Figures 2A, B, C, D, E and F). The thickness of the outer and inner epithelial surfaces of the ethmoidal conchae from both dog and goat was higher in the dog (41.5± 0.9 and 24.5 ±0.6) than in the goat (36.2±3.2 and 24.3±0.7 μm) (Table 1).
The olfactory epithelium consisted of ORNs, supporting and basal cells (Figure 2C, D). ORNs were mainly distributed in the mid-epithelial regions. Their nuclei were spherical in shape and pale basophilic-stained with dark eosinophilic cytoplasm. Numbers of ORNs in the outer olfactory epithelial surface was statistically higher in the dog (174.7±2.7) than in the goat (56.3 ±2.2) (Table 1). Supporting cells were columnar and had darkly basophilic stained oval to rod shaped nuclei with pale eosinophilic cytoplasm. The basal cells in dogs were consisted of two types of cells; horizontal with flat nuclei and globose with round nuclei, but, in the goat only globose cells were existed and were linearly arranged on the basement membrane. Additionally, apical acidophilic borders were clearly identified in the olfactory epithelium of both species (Figure 2C, D).
Table 1: Mean thickness of the outer and inner epithelial surface, lamina propria, numbers of ORNs in the outer olfactory epithelial surface from the ethmoidal conchae in both dog and goat. Values are means ± SE, (n=6).
| Animals / Parameters | Dog | Goat | p-value | |
| Thickness of the epithelium and Lamina propria (µm) | Outer epithelium | 41.5± 0.9 | 36.2±3.2 | 0.15 |
| Inner epithelium | 24.5 ±0.6 | 24.3±0.7 | 0.81 | |
| Lamina propria |
171±3.1b |
183 ±4.5a |
0.05 | |
| Numbers of ORNs in the outer olfactory epithelial surface |
174.7±2.7a |
56.3 ±2.2b |
0.000 | |
abMean values with different superscript letters in the same row are statistically significant from each other at level (p<0.05); N: number of animals.
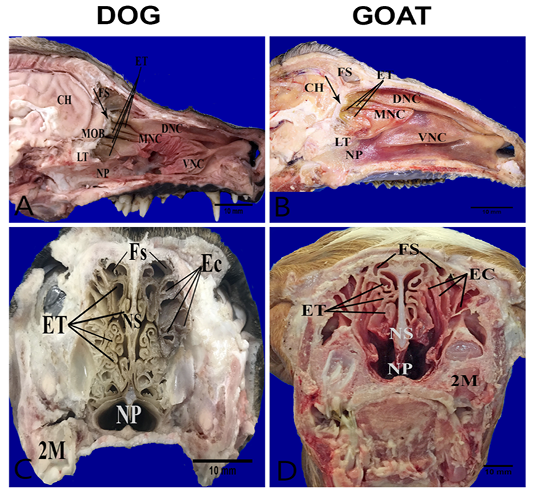
Figure 1: Anatomical features of the conchae in the nasal cavities of the heads’ dog (A, C) and goat (B, D). Parasagittal sections (A,B) showing the structures of the medial nasal wall, including dorsal nasal concha (DNC), middle nasal concha (MNC), ventral nasal concha (VNC), ethmoidal concha (ET), frontal sinus (FS), nasopharynx (NP), cribriform plate (arrow) ,lamina transversalis posterior (LT), main olfactory bulb (MOB) and cerebral hemisphere (CH). Transverse frozen sections (C, D) at the lateral canthi of eyes showing ectoturbinates (EC), endoturbinates (ET), frontal sinus (FS), nasal septum (NS) and second molar teeth (2M).
Within the propria toward the outer olfactory epithelial surfaces in both dog and goat, a large number of Bowman’s glands and sectioned nerve bundles were distributed unlike toward the inner epithelial surface contain fewer glands and nerve bundles only in the dog (Figure 2A, B). The Bowman’s glands were tubulo-acinar type with ducts that traverse through the olfactory epithelium. Their secretory cells were pyramidal in shape with oval basal nuclei (Figure 2C, D).
The nerve bundles tended to occur centrally within the lamina propria in the dog and basally in the goat. Axon nerve bundles within the propria were greater in diameter
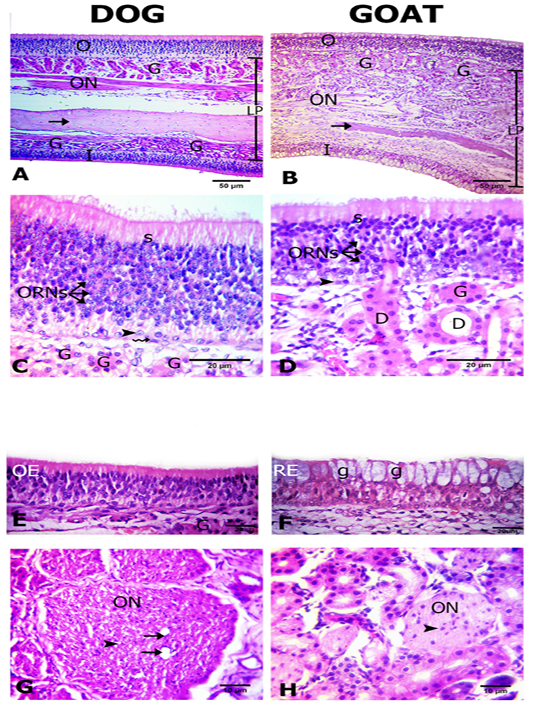
Figure 2: Histological features of the olfactory mucosae. Hematoxylin and eosin (H and E) stained sections from both dog (A) and goat (B) showing longitudinal sections of ethmoidal concha possess inner (I) and outer (O) epithelium and lamina propria (LP) with Bowman’s gland (G), olfactory nerve axon bundles(ON) and bone (arrows). The outer olfactory epithelium of both dog and goat (C, D) consists of supporting cells (S), olfactory receptor neurons (ORNs) and basal cells of horizontal (zigzag arrow) and globose (arrow heads) in shape. The ducts (D) of the Bowman’s glands (G) traverse through the olfactory epithelium (arrow). The inner epithelium (E, F) is olfactory (OE) in dog and respiratory (RE) with goblet cells (g) in goat. Cross sectional profiles in the lamina propria with larger size olfactory nerve bundles (ON) with blood capillaries (arrows) in dog (F) and smaller one in goat (G) contain the nucleus of the olfactory ensheathing cells (arrow heads).
in the dog than in the goat. The core of the nerve bundles in the dog possessed one to two blood capillaries. The axon bundles were encircled by a fibroblastic connective tissue sheath. The dark nuclei of olfactory ensheathing cells were enclosed the individual fascicles within the bundles (Figure 2G, H). There are variations in the thickness of lamina propria where it was significantly increased in the goat (183±4.5) than the dog (171±3.1 µm) (Table 1).
Immunohistochemical analysis
We studied the expressions of the GFAP and synaptophysin in the olfactory mucosa from both animal species (Figure 3).
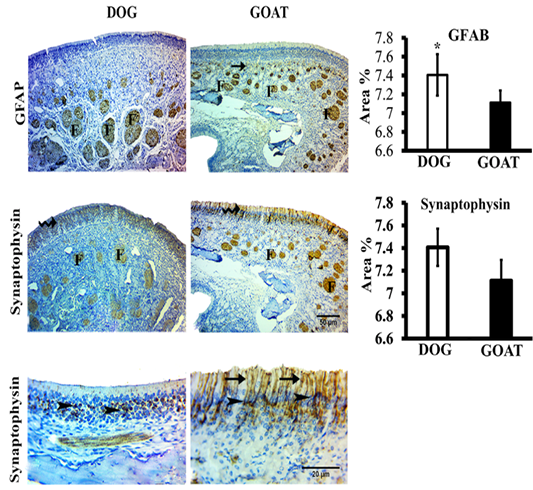
Figure 3: SImmunohistochemistry staining for GFAP and synaptophysin of the olfactory mucosa from both dog and goat. GFAP immunoreactions are deeply reacted in the nerve fascicles (F) of the distal and apical parts of underlying lamina propria in dog and goat, respectively. At the junction (arrow) between the olfactory epithelium and the initial parts of the olfactory nerves, reactions are less intense in the goat more than the dog. Synaptophysin immunoreactions are clearly expressed in the ORNs (zigzag arrow) and in the olfactory nerve fascicles (F) through the lamina propria in both species. Synaptophysin give a strong a perinuclear cap-like arrangement reaction (arrow heads) also, in the goat give a strong reaction in the apical dendrites (arrow). Brown color indicates a positive reaction. Bar charts demonstrating area % of the GFAP and Synaptophysin positive expressions in the olfactory mucosa of both dog and goat. Data are showed as means ±SE (n=6), * refer to statistically significant differences (p ≤ .05).
GFAP-immunostained sections revealed that, localizations of positive GFAP-immunoreaction in the olfactory mucosa of dog and goat was evident in the OECs of the olfactory nerve fascicles even the smallest cytoplasmic processes of
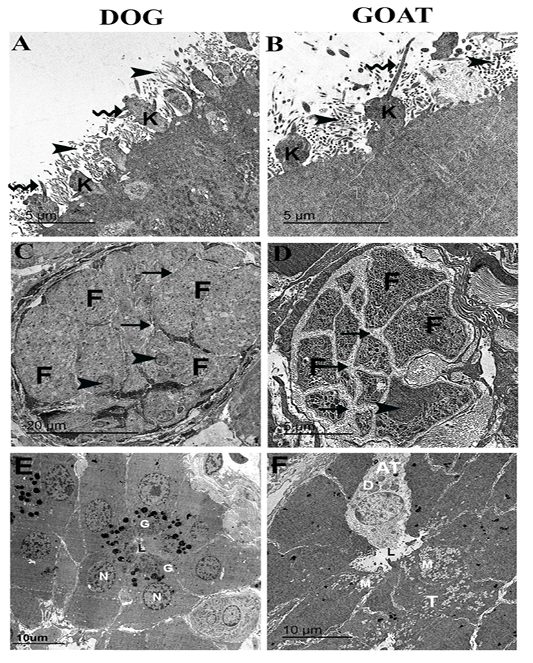
Figure 4: Ultrastructural features of the olfactory mucosa from both dog and goat (A-F). Features of the apical regions of the olfactory epithelium with cilia (zigzag arrow) that emanate from around the base in dog (A) and the tip in goat (B) of the olfactory knobs (k) and intermix with the microvilli (arrow heads) . A large olfactory axon bundle (C, D) with numerous fascicles (F) are wrapped and separated from each other by perineural-like sheath (arrows) of OECs processes with narrow (C) and wide (D) inter-fascicle separation and OECs nuclei (arrow heads). Cross section of the Bowman‘s gland acinus with serous cells (E) and mucous cells (F) with electron dense granules (G) and electron lucent coalescent granules (M) of the typical mucous cells (T) and electron dense granules (D) of A typical mucous cell (AT), lumen (L), nucleus (N).
these ensheathing cells. Statistically, the mean value of area percentage of the positively reacted GFAP in dog was 7.2±0.2 and showed a significant higher percentage than that of the goat 5.3±0.1.
Immunohistochemical detection of synaptophsin in the olfactory mucosa of both dog and goat. Positive synaptophsin -immunoreaction was in form of perinuclear cap-like arrangement in the olfactory receptor neurons also extended into the apical dendrites and into the axonal bundles of the olfactory nerve fascicles through the lamina propria, in the dog. Conversely, in the goat, a clearly strong reaction in the apical dendrites were detected. Statistically, the mean value of area percentage of synaptophysin immuno-positive reaction was non-significant in both dog (7.4±0.2) and goat (7.1±0.2).
Ultrastructure features of the olfactory mucosa
On the apical free borders of the olfactory epithelium of dog, ORNs were manifested by emanated cilia from around the bases of the knobs (Figure 4A), whereas in the goat, cilia of ORNs were emanated from the tips of the olfactory knobs to run parallel (Figure 4B).
Cross-sections in a large olfactory axon bundle with numerous fascicles in both dog and goat (Figure 4 A, B) revealed the presence of the unmyelinated axons of the olfactory nerves. In the dog, the fascicles were relatively more closely packed (narrow inter-fascicle separation) whereas, extensive processes of olfactory ensheathing cells surround the olfactory fascicles (wide inter-fascicle separation) were noticed in the goat (Figure 4 C, D). Cross-sections in the Bowman‘s gland acini in both dog and goat (Figure 4 E, F) showing the presence of serous acini a with numerous granules of varying denisities in the dog and typical mucous cells with electron lucent coalescent granules and A typical mucous cell with electron dense granules in the goat.
Our result showed that the ethmoidal complex provided a larger surface area for olfactory mucosa through the formation of highly folded scrolls and extended rostrally in the dog than in the goat where the complex was short and with few folds. The amplification of surface area by the lengthening and folding of the ethmoidal complex in the dog provide a larger surface for the ORNs which give confidence for the superior olfactory cues recorded in the dog (Kajiura et al., 2005; Schluessel et al., 2008).
Also, the ethmoidal complex consisted of endoturbinates and ectoturbinates that were 4 and 6, respectively in the examined dog. The similar result recorded by (Evans and De Lahunta, 2013). On the other hand, the investigated goat had 5-6 endoturbinates and 9-10 ectoturbinates which are in accordance with Singh et al. (1992). Endoturbinates and ectoturbinates were 7and 5-6 in young pig (Prakash et al., 2016), 15 and 4 in yak (Sharma and Gupta, 1991), 5 and 6 in sheep (Sharma et al., 1989; Ganganaik et al., 2004) and 6 and 8 in buffalo calves (Ganganaik et al., 2009a), respectively.
Another clear difference of the ethmoidal scrolls that were covered in outer and inner surface with olfactory mucosa in the dog but in the goat only the outer surface covered with olfactory mucosa. These findings are similar to those described in the goat (Kumar et al., 1993).
The olfactory epithelium gets a larger thickness in the dog than in the goat that is in accordance with previous studies reported by Kavoi et al. (2010) in dog and sheep. We notice a significantly greater increase in the number of ORNs of the dog than the goat. Based on these findings, we conclude that thickening of the epithelium in the dog is mainly due to the increase in ORNs numbers and so on increased the odor sensitivity like in rats (Apfelbach et al., 1991).
The structure of the olfactory epithelium was similar with the components in both dog and goat. This similarity is reported by (Kavoi et al., 2010; Kavoi, 2018) except that the dog has horizontal and globose basal cell and the goat possess only the globose one. These cells responsible for the ongoing process of neurogenesis in this epithelium as they serve to replace receptor and other epithelial cells lost during normal turn over or injury (Huard and Schwob, 1995).
Our result showed that the lamina propria contain a large number of Bowman’s glands. The latter were observed in the regions of the lamina propria subjacent to the outer mucosal epithelium of the ethmoidal conchae, unlike in the dog, they were observed in the outer and inner mucosal epithelium of the ethmoidal conchae that is in accordance with previous studies reported by Kumar et al. (1993). The types of the Bowman’s glands are varied in nature of secretion and the shape of secretory unit according to species and age variations. They were predominantly of the acinar type in neonate’s rabbits and of tubular type in the adult one (Kavoi et al., 2010). They are of mucous type in the investigated goat, as in horse (Kumar et al., 2000), sheep (Ganganaik et al., 2009b). They are of serous type in the investigated dog, goat (Kumar et al., 1993), buffalo (Gupta et al., 1994), camel (Suman et al., 1998).
The current study shows that the dog has a larger diameter of the olfactory axon bundle than goat. Similar data have been reported previously in the dog and sheep (Kavoi et al., 2010) and in neonate and adult rabbits (Kavoi et al., 2012). This proves that the dog has a functionally efficient olfactory mucosa as the size of a bundle is directly linked to the convergence proportion between olfactory mucosa axons and those of the olfactory bulb’s secondary neurons (Meisami, 1989).
Among the investigated dogs, blood capillaries current in the axonal bundle’s core. Similar findings were shown by (Kavoi et al., 2010; Kavoi et al., 2012) where high-thickness axon bundles have large diffusion distances that oxygen and nutrients have to cross in order to supply olfactory ensheathing cells within the bundle’s core or may be indicate of a feature of development.
GFAP immunopositive reactions were evident in the OECs of the olfactory nerve fascicles as in cat (Smithson and Kawaja, 2009) and not detected in guinea pig OECs that suggests a shift in the structural organization of the glial cytoskeleton (Smithson and Kawaja, 2009). ORNs can be histochemically detected in the investigated dog and goat by using antibodies against synaptophysin protein that continuously expressed in them to perform synaptic contact with their target, the olfactory glomeruli (Bergmann et al., 1993).
The cilia of ORNs emanated from the tips of the knobs to run parallel in the form of a bundle in the goat. These results recorded in neonates’ rabbits (Kavoi et al., 2012), whereas in the dog, the cilia emanated from around the bases of the knobs, seems to be associated with higher cilia numbers as in the horse (Kumar et al., 2000), adult rabbits (Kavoi et al., 2012) and rufous sengi (Kavoi, 2018).
In conclusion, our study demonstrated that the increased size of the bundle fascicles with narrow interfascicular gaps in the dog where the goat with wide interfascicular gaps may be associated with the higher olfactory nerve concentrations and hence the higher convergence to the central relay neurons thereby resulting in improved olfactory sensitivity.
Therefore, our data revealed structural difference of the olfactory mucosa with more developed structures in dog that could cover the goat.
Authors Contribution
All authors equally contributed to this article.
List of abbreviation
DAB: Diaminobenzidine; GFAB: Glial Fibrillary Acidic Protein; OECs: Olfactory Ensheathing Cells; ORNs: Olfactory Receptor Neurons; PBS: Phosphate Buffer Saline; TEM: Transmission Electron Microscopy.
Conflict of interest
There is no conflict of interest.
References






