Advances in Animal and Veterinary Sciences
Case Report
Pleomorphic Variant of Leydig Cell Tumor in a Dog
Soo-Hyeon Kim, Byung-Joon Seung, Seung-Hee Cho, Ha Young Lim, Jung Hyang Sur*
Department of Veterinary Pathology, Small Animal Diagnostic Center, College of Veterinary Medicine, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul, Korea.
Abstract | Leydig cell tumor is relatively common in dogs, accounting for approximately a quarter to half of all canine testicular tumors. While most Leydig cell tumors are benign, malignant forms have been reported. A 13-year-old Pomeranian visited a local hospital with a chief complaint of edema in the left testicle. Radiography and ultrasonography revealed a mass in the left testicle; therefore, orchidectomy was performed. Microscopic analyses revealed the growth of a neoplastic mass compressing adjacent seminiferous tubules. Immunohistochemistry confirmed that the neoplasm was derived from Leydig cells. Neoplastic cells showed marked cellular and nuclear pleomorphisms, which is one of criteria for malignant Leydig cell tumor. However, the immediate postsurgical outcome was good, and seven months after surgery, the patient recovered well without any complications or recurrence. Therefore, the present study indicates that cellular and nuclear polymorphisms cannot be alone inform the differentiation between benign and malignant Leydig cell tumor in dogs.
Keywords | Dog, Leydig cell tumor, Testicular tumor, Diagnostic pathology, Histopathology
Received | September 17, 2019; Accepted | December 26, 2019; Published | January 22, 2020
*Correspondence | Jung-Hyang Sur, Professor, DVM, Department of Veterinary Pathology, Small Animal Diagnostic Center, College of Veterinary Medicine, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul, Korea 05029; Email: [email protected]
Citation | Kim S-H, Seung B-J, Cho S-H, Lim HY, Sur JH (2020). Pleomorphic variant of leydig cell tumor in a dog. Adv. Anim. Vet. Sci. 8(2): 147-150.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.2.147.150
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Kim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Testicular tumor is common in both canine and human species (Grieco et al., 2008). Leydig cell tumor (interstitial cell tumor), sex cord-stromal tumor derives from testicle, presents relatively more frequently among in dogs (Hayes et al., 1976; Liao et al., 2009) while incidence in human is rare (Nason et al., 2018). Indeed, several reports indicate that Leydig cell tumor accounts for a quarter to half of all testicular tumors in the canine species (Hayes et al., 1976; Liao et al., 2009). Although most Leydig cell tumors are known benign, few reports have observed canine malignant Leydig cell tumor that had even metastasized to distant organs (Canadas et al., 2016; Togni et al., 2015; Kudo et al., 2019). Even in malignant cases with distant metastasis, neoplastic Leydig cell tumors have presented low to moderate cellular polymorphisms (Canadas et al., 2016; Togni et al., 2015; Kudo et al., 2019). By contrast, herein we report untypical, benign Leydig cell tumor with significant cellular polymorphism.
A 13-year-old intact male Pomeranian visited a local animal hospital with clinical signs of enlargement of the left testicle. In preoperative evaluation, the dog had perineal hernia and atrophied right testicle. Ultrasonography revealed a hyperechoic mass and free fluid with high echogenicity in the left scrotum. In addition, herniated contents investigated through ultrasonography included colon, prostate and urinary bladder. Computed tomography images evinced a mass with a 1-cm diameter within the left scrotum and atrophy of right testicle (Figure 1). Orchidectomy for both testicles was performed. A large quantity of serous fluid and a neoplastic mass was observed in the tunica vaginalis of the testis (Figure 2). In addition, a splenic nodule was identified on pre-surgical physical examination. Splenectomy with orchidectomy was conducted.
The testicular sample collected from the orchidectomy was fixed in 10% neutral buffered formalin, embedded in paraffin wax, sliced into 4µm sections, and stained with hematoxylin and eosin (H and E). Immunohistochemistry was performed to differentiate the Leydig cell tumor from other testicular tumors. Slides were incubated with 3% hydrogen peroxide for 20 minutes at room temperature, and washed with phosphate buffered saline (PBS) for three times. The slides were then dipped into Tris-EDTA solution (pH 9.0) and heat-induced antigen retrieval was conducted with a microwave (750W). The slides were cooled down, and incubated with 5% normal goat serum for 30 minutes to remove non-specific signals, and then with primary antibodies (Table 1), 4 oC, overnight.
Table 1: Primary antibodies used for the differentiation of leydig cell tumor.
| Primary antibody | Clone | Antigen retrieval | Dilution |
| Vimentin | V9 | Tris-EDTA, 10 min | 1:800 |
| c-kit (CD117) | Polyclonal | Tris-EDTA, 15 min | 1:300 |
| MelanA | A103 | Tris-EDTA, 15 min | 1:100 |
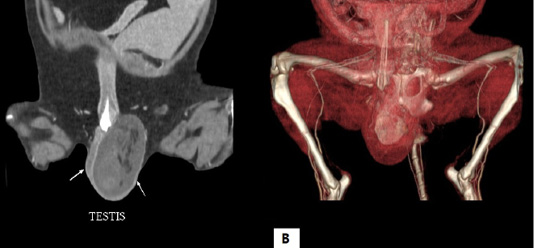
Figure 1: Computed tomography scan of the testicle. (A) Two-dimensional computed tomography scan shows hyperdensity of left testicle and (B) three-dimensional computed tomography scan presents a mass within the left testicle.
The slides were washed with PBS for three times, and incubated for 40 minutes at room temperature with a peroxidase-conjugated polymer, which contains antibodies to rabbit/mouse immunoglobulins. The slides were washed for three times with PBS once more, visualized by 3’3-diaminoobenzidine (DAB), and counterstained with Gill’s hematoxylin.
Microscopically, a neoplastic mass was observed in the left testicle, which proliferated to the extent that it compressed adjacent atrophic seminiferous tubules. A neoplastic mass was encapsulated by fibrous connective tissue, and demonstrated no invasive growth to adjacent tissues. In the mass and adjacent testicular structures, there were multifocal hyperemic lesions. Neoplastic Leydig cells (interstitial cells) organized solid sheet, and had indistinct cell borders, moderate amount of eosinophilic cytoplasm with variable accumulations of lipids, marked nuclear polymorphisms, anisokaryosis, and distinct single nucleoli (Figure 3).
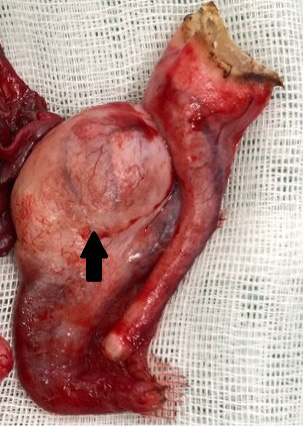
Figure 2: Gross lesion of the surgically removed tissue. A neoplastic mass with a diameter of 1 centimeter was observed in the tunica vaginalis (arrow).
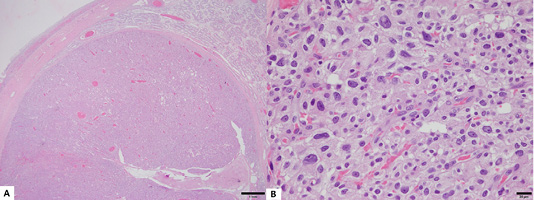
Figure 3: Histopathological lesion of the tumor. (A) An encapsulated neoplastic mass has proliferated and compresed adjacent atrophic seminiferous tubules (H&E stain, Bar = 1mm). (B) Neoplastic cells showed marked nuclear pleomorphism, anisokaryosis, and distinct single nucleolus (H&E stain, Bar = 20 µm).
There was less than 1 mitotic count per 10 high-power fields, and no evidence of invasion in adjacent lymphatic and blood vessels. Immunohistochemical analysis revealed that neoplastic cells showed homogenous positivity for vimentin, c-kit (CD117), and Melan A, confirming that neoplastic cells were derived from Leydig cells (Figure 4). The splenic mass was diagnosed as lymphoid nodular hyperplasia, and evidence for metastasis of the Leydig cell was not found. The immediate postsurgical outcome was good. The patient showed stable vital signs, and the patient recovered by 7 months following surgery without recurrence or any complications.
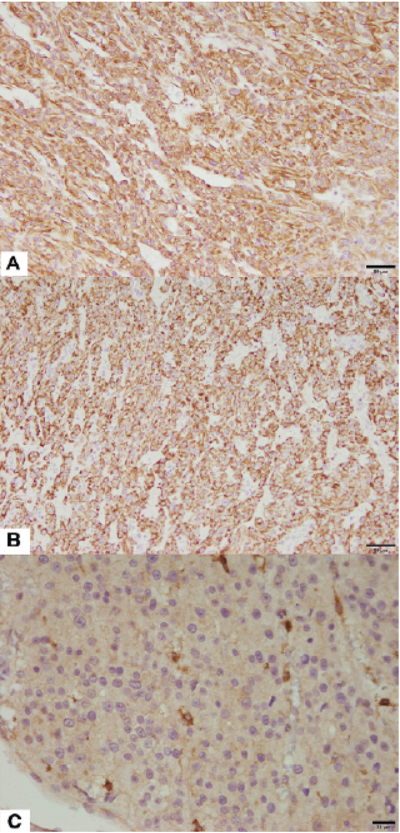
Figure 4: Immunohistochemistry for definite diagnosis of the Leydig cell tumor. Neoplastic Leydig cells showed homogenous positivity for (A) vimentin (Counterstained with Mayer’s hematoxylin, Bar = 50 µm), (B) Melan A (Counterstained with Mayer’s hematoxylin, Bar = 50 µm), and (C) c-kit (CD117) (Counterstained with Mayer’s hematoxylin, Bar = 20 µm).
Although several studies have described variant forms of Leydig cell tumor in rats (Qureshi et al., 1991; Rehm and Waalkes, 1988), reports showing variable histological patterns for the canine incidence of this tumor are lacking. Even though most Leydig cell tumors are reportedly benign, there is evidence for their malignant counterparts. In most cases, cellular and nuclear shapes of neoplastic cells composing Leydig cell tumors are known to be small and regular (Agnew and Maclachlan, 2017). Histologic criteria for the diagnosis of malignant Leydig cell tumor is based on the irregularity of neoplastic cells, numerous mitotic counts, and vascular invasion (Agnew and Maclachlan, 2017). However, cellular polymorphism and anisokaryosis were not prominent in previous studies reporting malignant Leydig cell tumor with metastasis (Canadas et al., 2016; Kudo et al., 2019; Togni et al., 2015), in contrast to the present study. Instead, those previous studies showed relatively high mitotic count (6-21 or common in 10 high power field), whereas mitotic count was low in the present case (<1 in 10 high power field).
The present study indicates that Leydig cell tumor with severe nuclear pleomorphism could exhibit benign behaviour, thus showing uncommon histologic variant of benign tumor. The evidence provided herein indirectly proves that nuclear and cellular polymorphisms cannot alone indicate malignant interstitial cell tumor in canine species, and suggests mitotic count could be additional criteria to differentiate malignant Leydig cell tumor from its benign counterparts. However, long-term follow-up is necessary to confirm the benign feature of the tumor in the present study.
Acknowledgements
The authors thank Mr. Y-S. Yoon (Shine veterinary clinics) for providing clinical data and tissue samples. This work was supported by the Bio and Medical Technology Development Program of the NRF funded by the Korean government, MSIT [grant number 2016M3A9B6903437].
Authors Contribution
SHK collected clinical data, drafted and revised the manuscript. BJS, SHC, HYL interpreted the clinical and histopathological data. JHS designed the project and critically revised the manuscript.
Conflict of interest
The Authors declare that there is no conflict of interest.
References






