Advances in Animal and Veterinary Sciences
Research Article
Effects of Dietary Bacillus coagulans D3372 Supplementation as Probiotics on Broiler Performance, Ileal Microflora, Meat Quality, Nutrient Retention, and Metabolizable Energy
Zainuddin, Arif Darmawan, Sumiati*, Komang Gede Wiryawan, Nahrowi
Department of Nutrition and Feed Technology, Faculty of Animal Science, IPB University, 16680 Bogor, West Java, Indonesia.
Abstract | A study was carried out to investigate the impacts of supplemental diets with Bacillus coagulans D3372 as a probiotic (BCP) on broiler performance, ileal microflora, meat quality, nutrient retention, and metabolizable energy. Six hundred one-day-old chicks were randomly allotted in 4 treatments with 5 replicates (30 birds in each replication with similar ratio of male and female) for 35 days. Dietary treatments were (T0) basal diet (BD) without BCP, (T1) BD + 105 CFU of BCP/g of diet, (T2) BD + 106 CFU of BCP/g of diet, and (T3) BD + 107 CFU of BCP/g diet. The BCP supplementation of broiler diets improved the body weight gain and reduced the feed conversion ratio at the final of experimental periods (P<0.05), compared with that fed T0. Nevertheless, the inclusion of probiotics had no effects on ileal Lactobacillus and E. coli counts of broilers (P>0.05). Chicks fed probiotics had a significant reduction of carcass cholesterol levels (P<0.05) than that fed T0 diet. Addition of BCP in broiler diets markedly enhanced protein (P<0.05) and fat (P<0.05) retentions compared with those of the T0 group. However, BCP supplementation significantly improved true metabolizable energy (P<0.05) and nitrogen-corrected metabolizable energy (P<0.05) of broilers. This suggests that dietary addition with BCP can promote growth performance by improving the protein retention, fat retention, and metabolizable energy utilizations of broiler chickens, and reduce cholesterol levels of the carcass, with no impact on ileal microflora.
Keywords | Broiler, Performance, Probiotic, Metabolizable energy, Nutrient retention
Received | June 25, 2019; Accepted | August 19, 2019; Published | January 03, 2020
*Correspondence | Sumiati, Department of Nutrition and Feed Technology, Faculty of Animal Science, IPB University, 16680 Bogor, West Java, Indonesia; Email: [email protected]
Citation | Zainuddin, Darmawan A, Wiryawan KG, Nahrowi (2020). Effects of dietary bacillus coagulans d3372 supplementation as probiotics on broiler performance, ileal microflora, meat quality, nutrient retention, and metabolizable energy. Adv. Anim. Vet. Sci. 8(1): 115-123.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.1.115.123
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Sumiati et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The gut of newly hatched chicks is immature and very sensitive to pathogenic microorganisms. Ingested feed and water will develop the intestinal function and its microflora. Enteric diseases are a vital concern for the poultry industry, causing lost productivity, increasing mortality, and the associated contamination of poultry products for human consumption (Patterson and Burkholder, 2003). Antibiotic growth promoters are often applied to remove the destructive microbes of the gastrointestinal tract (GIT) for improving growth and feed efficiency. However, the use of antibiotic has disadvantages such as antibiotic residue and resistant pathogenic bacteria. Therefore, antibiotic application as growth promoters in the animal feed has been restricted in Indonesia. Based on that, it is necessary to find alternative ways to replace the utilization of antibiotics in animal production. Probiotic is one way that can be used for poultry production.
Generally, directly fed microbial tend to improve the bacterial culture ability for modulating gut microflora, which is able to modify the GIT environment in positive properties, support beneficial bacteria, and stimulate the growth performance and feed efficiency of broilers (Lei et al., 2014). In contrast with mostly known probiotics, which are susceptible and incapable to survive at high temperatures during feed processing; spore-forming probiotics are metabolically dormant, and very resilient to external circumstances such pH and temperature extremes (Nicholson, 2002) such as B. subtilis (Zhang and Kim, 2014), B. coagulans (Xu et al., 2017), and B. amyloliquefaciens (Ahmed et al., 2014; Lei et al., 2014) that have been widely applied as a probiotic in poultry feed. Bacillus is an aerobic microbe, capable to form endospores, able to survive and germinate in the GIT, and can excrete through the feces (Shivaramaiah et al., 2011). Bacillus spp. produce extracellular enzymes that may enhance digestibility and absorption of nutrients, and able to modulate immune function of the gut (Jeong and Kim, 2014). The B. coagulans characteristics have the sporulated capacity, easily cultured in bulk, and more resistant to heat that facilitates the pelleting process in the endospore forms (Zhou et al., 2010).
The beneficial effects of probiotic diet supplements on broiler performance parameters were reported by a number of previous studies (Wang and Gu, 2010; Jeong and Kim, 2014). Supplementation diets with B. coagulans significantly increased growth performance and feed efficiency of broiler chicks (Zhou et al., 2010). Protease and amylase were significantly improved in broilers fed diets supplemented with B. coagulans NJ0516 (Wang and Gu, 2010). The dietary addition with 200 mg of B. coagulans kg-1 markedly increased broiler performance, immunity organ index and duodenal villus height (Xu et al., 2017). Therefore, this study was conducted to determine the influences of supplementation diets with B. coagulans D3372 (BCP) on growth performance, ileal microflora, meat quality, nutrient retention, and metabolizable energy of broiler chicks kept in a postal open house under natural circumstances.
MATERIALS AND METHODS
Experimental Birds and Diets
Six hundred one-day-old male and female Lohmann broilers with initial body weight of 40.80±0.18 g were obtained from a local commercial hatchery, which were vaccinated Newcastle disease and infectious bursal disease. Broilers randomly allotted into 4 treatments with 5 replicate pens with 30 birds each pen (15 male chicks and 15 female chicks). Each replicate was allocated to a clean floor pen (4 m2) and birds were raised on a rice husk litter in a postal open house under natural circumstances. The heater was provided with 100 W bulb per pen for 14 days.
The experimental diets were labeled as follows: basal diet (BD) without BCP supplementation (T0/Control), BD containing BCP concentration of 105 CFU/g of diet (T1), BD containing BCP concentration 106 CFU/g of diet (T2), and BD containing BCP concentration of 107 CFU/g of diet (T3). The BCP manufactured by Kyushu Medical, Co., Ltd., Japan. The BD was in crumble form and was formulated for pre starter (1 to 7 days), starter (8 to 21 days), and grower (22 to 35 days) phases of broilers and its ingredient is shown in Table 1. Experimental diets and water were available ad libitum. The BD was adjusted to the chicken growth phase requirement in the high nutrient density diets (Leeson and Summers, 2005).
Table 1: Ingredient and nutrient composition of basal diets
| Ingredient (%) | Prestarter | Starter | Grower |
| (1 - 7 days) | (8 - 21 days) | (22 - 35 days) | |
| Corn | 58.70 | 56.00 | 59.00 |
| Rice bran | 0.00 | 4.50 | 6.65 |
| Soybean meal | 11.18 | 21.40 | 15.60 |
| Meat and bone meal | 13.00 | 7.60 | 7.00 |
| Corn gluten meal | 11.40 | 5.25 | 6.50 |
| Crude palm oil | 4.00 | 3.00 | 3.00 |
| Calcium carbonate | 0.20 | 0.50 | 0.50 |
| Sodium chloride | 0.20 | 0.20 | 0.20 |
|
Premix1 |
0.50 | 0.50 | 0.50 |
| DL-Methionine | 0.22 | 0.40 | 0.40 |
| L-Lysine | 0.40 | 0.45 | 0.45 |
| Tryptophane | 0.10 | 0.10 | 0.10 |
| Probiotic | 0.10 | 0.10 | 0.10 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient composition | |||
|
Dry matter2 |
91.51 | 90.88 | 84.17 |
|
Metabolizable energy, kcal/kg3 |
3200 | 3050 | 3100 |
|
Crude protein2 |
23.64 | 22.55 | 19.14 |
|
Extract ether2 |
6.93 | 5.02 | 2.28 |
|
Crude fiber2 |
0.56 | 1.52 | 2.94 |
|
Methionine3 |
0.61 | 0.69 | 0.69 |
|
Lysine3 |
1.17 | 1.32 | 1.16 |
|
Methionine + Cystine3 |
0.91 | 0.97 | 0.93 |
|
Ash2 |
7.82 | 7.75 | 7.53 |
|
Calcium2 |
1.66 | 1.15 | 0.92 |
|
Available-P2 |
0.78 | 0.57 | 0.54 |
|
Sodium Chloride2 |
0.46 | 0.40 |
0.27 |
1Premix kg-1: Vitamin A 1 250 000 IU, Vitamin D 250 000 IU, Vitamin E 750 IU, Vitamin K3 200 mg, Vitamin C 5 000 mg, Vitamin B1 250 mg, Vitamin B2 400 mg, Vitamin B6 100 mg, Vitamin B12 1.2 mg, Biotin 20 mg, Folic acid 50 mg, Nicotinic acid 3 000 mg, Calcium-D-panthotenate 400 mg, Choline chloride 1 500 mg, Copper 500 mg, Iron 2 500 mg, Iodine 20 mg, Manganese 6 000 mg, Selenium 20 mg, Zinc 7 000 mg, Cobalt 20 mg, Zinc Bacitracine 2 100 mg, Lysine 16 000 mg, DL-Methionine 5 000 mg, Threonine 4 000 mg, Antioxidant 800 mg.2 Based on laboratory analysis
3 Based on calculated
Broiler Performance
The birds were weighed when arrived from the hatchery to the experimental farm as an initial weight. Growth performance parameters were weekly obtained such as body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR) defined as FI:BWG (g:g). Overall BWG, FI, and FCR were calculated during the observation periods.
Mortality Rate and European Production Efficiency Factor
The mortality rate was completely recorded at each treatment and accumulated during the experimental periods. The percentage of mortality was calculated by dividing the total mortality to the initial population of each treatment. The European production efficiency factor (EPEF) is closely related to BW gain, live ability and FCR, as follows (Awad et al., 2009):
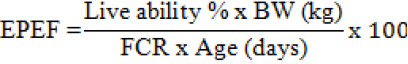
Ileal Microflora Counting
The ileal microbe enumeration procedure was conducted with slightly modification (Ahmed et al., 2014; Cengiz et al., 2015). Briefly, chickens were exsanguinated at day 35 of age, and de-feathered, opened, eviscerated manually, and the GIT was removed from the carcass under aseptic conditions. Small intestine was ligated on the distal of the gizzard to the cecum bifurcation point. An approximately 1 g of the ileal digesta was aseptically collected into a sterile tube containing 9 mL of a buffered peptone water solution (M614® HiMedia Laboratories Pvt. Ltd. A-516, India), was subsequently diluted to 10-9. Viable contents of bacteria in the ileal digesta samples were conducted by plating serial 10-fold dilutions in duplicate. The MRS Broth agar (M1164®HiMedia Laboratories Pvt. Ltd. A-516, India) was applied to isolate Lactobacillus spp. incubated anaerobically at 37 °C for 48 hours. An EMB agar (M022S®HiMedia Laboratories Pvt. Ltd. A-516, India) was used to isolate Escherichia coli incubated aerobically at 37 °C for 24 hours. The determination of bacterial colony forming unit (CFU) was carried out after removal of the incubator and calculated as log 10 CFU mL-1.
Meat Quality Assay
Carcass samples with skin of male birds were collected for meat quality measurement at the 35 days of age, filleted and homogenous grinded. Two g of meat samples were extracted by 10 ml of diethyl ether. The enzymatic determination of total cholesterol was modified using the chod-pap method (Allain et al., 1974), an enzymatic colorimetric test for cholesterol with lipid clearing factor using cholesterol kit (Cholesterol liquid color 10017® HUMAN, Germany). Total cholesterol was measured using a spectrophotometer reader (Hitachi U2001 UV VIS, Japan) at 500 nm. The carcass fatness was quantified by the extraction Soxhlet method (AOAC, 1990).
Nutrient Retention and Metabolizable Energy Analyzes
The procedure of digestibility analysis was conducted with slight modification (Farrell, 1978). The male broiler chickens used in this trial consisted of 20 birds fed experimental diets, and 5 birds were no fed experimental diets (fasted from feed) for endogenous energy (EE) measurement. All birds located in a metabolic cage individually and adapted for 3 days. All chickens (25 birds) were fasted from feed for 24 hours, and drinking water was available ad libitum. Furthermore, a total of 20 broilers were fed test diets, and 5 chickens were refasted for 24 hours, water was given ad libitum. The excreta of each chicken was accommodated with a tray and sprayed with 0.01% H2SO4 solution every 2 hours during the collection periods to bind the nitrogen. Excreta was weighed and kept in the freezer for 24 hours to prevent decomposition. All samples were thawed, dried in an oven 60o C for 48 hours, milled and cleaned from small feathers, and weighed. Feed and excreta analyses for dry matter, ether extract, and crude protein were determined using AOAC (1990) procedure. The gross energy of feed and excreta were measured by an adiabatic calorimeter bomb (Parr 1261® Bomb Calorimeter, Parr Instrument Company, Illinois). Retention of protein and fat, as well as metabolizable energy were calculated by Sibbald and Wolynetz (1985).
Statistical Analysis
Experimental data were analyzed in a completely randomized design by General Linear Model - Analysis of Variance procedures. Significant differences among the treatment groups were determined using Duncan’s multiple range test at p<0.05. Statistical analysis was conducted by SPSS for Windows statistical package program, version 16.0.
RESULTS
Broiler Performance
The initial BW of chicks did not differ between the experimental treatment groups (40.83±0.18 g). The broiler performance at various growth phases fed diets containing BCP during the experimental periods is shown in Table 2. The addition of BCP in broiler diets had a positive effect on BWG in pre-starter phase (P<0.05), but had no impact on FCR (P>0.05). BWG was significantly low, and FCR was poor in broilers offered T0. Supplemental diets with BCP increased markedly BWG (P<0.05) in starter phase without effect on FCR and feed ingestion. In the grower
Table 2: Direct fed microbial effects with BCP on growth performance of the experimental birds.
| Parameter | Treatment | |||||
| T0 | T1 | T2 |
T3 |
|
||
| Feed intake (g/bird) | ||||||
| Prestarter | 128±3.7 | 129±3.3 | 128±4.8 | 127±3.2 | ||
| Starter | 898±5.6 | 918±15.8 | 909±4.3 | 888±12.4 | ||
| Grower | 1796±27.5 |
1773±34.6 |
1774±35.4 | 1780±43.4 | ||
| Total | 2820±27.5 | 2829±37.3 | 2811±33.5 | 2788±54.1 | ||
| Body weight gain (g/bird) | ||||||
| Prestarter |
120±2.1bc |
135±2.0a |
125±1.5b |
116±1.9c |
||
| Starter |
672±15.1c |
735±3.1a |
696±13.0b |
679±15.2c |
||
| Grower |
805±27.3c |
885±33.5a |
846±30.3b |
780±13.3c |
||
| Total |
1597±18.3c |
1756±36.5a |
1664±42.2b |
1574±19.4c |
||
| Feed conversion ratio (FCR) | ||||||
| Prestarter | 1.04±0.04 | 1.02±0.02 | 1.05±0.01 | 1.03±0.02 | ||
| Starter |
1.34±0.03a |
1.25±0.02b |
1.30±0.03ab |
1.31±0.02ab |
||
| Grower |
2.24±0.05ab |
2.01±0.04c |
2.10±0.05bc |
2.28±0.05a |
||
| Total |
1.76±0.01a |
1.61±0.02c |
1.69±0.03b |
1.77±0.02a |
||
Prestarter (day 1-7), Starter (day 8-21), Grower (day 22-35), and Total (day 1-325).
The results are reported as means ± SEM (150 birds per treatment).
a,b Means with different superscripts are significantly different within the same row, (P<0.05, ANOVA, Duncan’s test).
Table 3: BCP supplementation impacts on mortality rate (%) and European production efficiency factor (EPEF) of the experimental birds.
| Parameter |
Treatment |
|||
| T0 | T1 | T2 | T3 | |
| Mortality (%) | ||||
| Prestarter | 1.3 | 0.7 | 0.0 | 1.3 |
| Starter | 0.7 | 0.7 | 1.3 | 0.7 |
| Grower | 2.0 | 1.3 | 1.3 | 1.3 |
| Total | 4.0 | 2.7 | 2.7 | 3.3 |
| EPEF | ||||
| Prestarter |
221±9.9b |
248±9.3a |
222±3.6b |
216±2.9b |
| Starter |
297±11.2c |
348±4.9a |
314±11.4b |
304±9.5b |
| Grower |
205±5.3c |
252±12.4a |
230±11.3b |
200±3.3c |
| Total |
259±2.6c |
312±11.4a |
285±12.6b |
257±4.0c |
Prestarter (day 1-7), Starter (day 8-21), Grower (day 22-35), and Total (day 1-325).
1The results are reported as means ± SEM (150 birds per treatment).
a,b Means with different superscripts are significantly different within the same row, (P<0.05, ANOVA, Duncan’s test).
phase, the BCP diets reduced significantly FCR (P<0.005), with the highest observed results in T1 group. Overall, BCP supplementation enhanced significantly (P<0.05) BWG and reduced FCR compared to control group, and did not affect on FI. The highest value was in T1 during the observation periods.
Mortality Rate and European Production Economic Factor
The mortality rate of broilers was not affected by dietary BCP (Table 3). The probiotic inclusion of broiler diets improved significantly European production economic factor (EPEF) during the experimental periods (Table 3). Birds fed probiotic were higher EPEF than the control group, especially in T1 was markedly higher compared with that fed T2, T3 and T0 at pre-starter (P<0.05), starter (P<0.05),
Table 4: Effects of BCP supplementations on ileal digesta microflora counts (Log10 CFU/g) and meat quality of male birds at day 35 of age.
| Parameter | Treatment | |||
| T0 | T1 | T2 | T3 | |
| Ileal microflora | ||||
|
Lactobacilus spp. |
9.29±0.13 | 8.77±0.21 | 8.80±0.45 | 9.18±0.22 |
|
E. coli |
7.23±0.46 | 8.44±0.24 | 7.53±0.45 | 7.51±0.37 |
| Carcass | ||||
| Cholesterol, mg/100 |
99±8.3a |
76±5.74c |
84±4.8b |
80.±8.2b |
| Carcass fatness, % (g/kg) | 6.2±0.4 | 7.6±0.7 | 7.5±1.2 | 9.3±0.8 |
1The results are reported as means ± SEM (5 birds per treatment).
a,b Means with different superscripts are significantly different within the same row, (P<0.05, ANOVA, Duncan’s test).
Table 5: Supplemental diets with BCP on protein and fat retentions (%), and metabolizable energy (as fed) of male birds.
|
Treatment |
||||
| Parameter |
T0 |
T1 |
T2 |
T3 |
| Retained nutrient (%) |
|
|
|
|
| Protein |
78.7±1.5b |
82.4±1.2a |
80.6±1.1a |
78.9±1.0b |
| Fat |
77.3±1.6b |
81.3±1.4a |
80.7±1.7a |
77.5±1.4b |
|
Metabolizable energy (kcal/kg diet)2 |
|
|
||
| AME |
3074±26c |
3210±34a |
3186±36b |
3016±50c |
| AMEn |
3066±26c |
3202±35a |
3177±35b |
3006±47c |
| TME |
3296±25b |
3388±33a |
3380±40a |
3201±49c |
| TMEn |
3288±25b |
3380±33a |
3371±41a |
3192±54c |
1 The results are reported as means ± pooled SEM (5 birds per treatment).
2 AME: apparent metabolizable energy, AMEn: nitrogen-corrected apparent metabolizable energy, TME: true metabolizable energy, TMEn: nitrogen-corrected true metabolizable energy
a,b Means with different superscripts are significantly different within the same row, (P<0.05, ANOVA, Duncan’s test).
and grower (P<0.05) phases. Regarding the value of EPEF, this have correlated to utilize the nutrients efficiently for improving BW and FCR of birds.
Ileal Microflora
The effects of BCP supplementation on ileal digestive microflora numbers of male broilers at day 35 of age are presented in Table 4. The BCP diets had no influences on ileal Lactobacillus and E. coli counts. No difference between groups was observed on ileal microflora contents of male birds. Intestinal microbes were tightly regulated by the host to keep the microbial homeostatic in the gut.
Meat Quality
Probiotic supplementation reduced significantly carcass cholesterol levels (P<0.05) compared with those the control treatment (Table 4). The lowest value was observed in T1 group than the other groups. Cholesterol homeostasis was regulated mainly by the liver and by the gut of chickens. The intestine regulated the absorption and excretion of cholesterol diets and bile salts excretion in the feces. Probiotics diets provided a hypocholesterolemic impact by modulation activity of these microbes on the liver and intestine of the birds.
Nutrient Retention and Metabolizable Energy
The BCP supplementation of broiler diets improved significantly protein retention (P<0.05) in broiler compared with those fed T0 diets (Table 5). The addition of T1 diet resulted in the highest protein retention compared to the other groups. The ability of broiler chickens to utilize nutrients increased fat retention (P<0.05). Broilers fed T1 and T2 diets had higher fat retention than that fed T0 diet.
Supplemental diets with BCP had no impact on apparent metabolizable energy (AME) (P>0.05) and nitrogen-corrected apparent metabolizable energy (AMEn) (P>0.05). Probiotics enhanced significantly true metabolizable energy (TME) (P<0.05) and nitrogen-corrected true metabolizable energy (TMEn) (P<0.05). Chickens fed T1 were greatest in AME and AMEn values. The highest values of TME and TMEn were obtained in broilers fed T1 and T2 diets (Table 5). There was estimated that the efficacy of probiotics inclusion in broiler diets to encourage broiler performance by gastrointestinal status enhancement for improving nutrient digestibility, and modulating broiler health.
DISCUSSION
The diets played the main role to provide the nutrient for metabolizable requirements and modulated various functions of the body. In this work, optimizing growth performance and feed efficiency in broiler chickens were a consequence of probiotic inclusion effects in diets could maintain favorable microbial counts (Patterson and Burkholder, 2003), improve nutrient digestibility (Lei et al., 2014; Zhang and Kim, 2014), and might change histomorphometry in the small intestine of broilers (Awad et al., 2009; Song et al., 2014). There have supported better intestinal integrity and functions, and growth performance enhancement. Dietary supplementation of probiotics lead to improve the villus height and crypt depth of intestinal mucosa in broilers (Awad et al., 2009; Song et al., 2014). Higher villi suggests an enlarged surface area capability for greater absorption of available nutrients in small intestine (Caspary, 1992), but deeper crypts indicate fast tissue turnover to permit renewal of the villus as required for normal responses of sloughing or inflammation from pathogens (Yason et al., 1987). However, smaller villi and deeper crypts may lead to poor nutrient absorption, increase secretion in the gastrointestinal tract, and lower performance (Xu et al., 2003). Overall, there has been suggested that broiler performance could be improved effectively by supplemental diets with BCP.
Probiotics played a vital role to keep the host intestinal integrity by modulating microbial ecosystem, improving mucosal barrier, promoting immune system, and enhancing broiler health (Ahmed et al., 2014; Song et al., 2014; Zhang and Kim, 2014). The ileal Lactobacilli and E. coli counts of male broilers had no significant effects at day 35 of age. Dietary supplementation with B. amyloliquefaciens was not significant influence on Lactobacillus and Bacillus populations of the cecal digesta of male birds at day 35 of age; however, E. coli number had a significant decline of broiler ceca, with the lowest value by the administration of 20 g/kg of probiotics (Ahmed et al., 2014). The broiler diets with probiotics were not impact the total aerobes, Salmonella, and Lactobacilli contents of ileal broilers (Cengiz et al., 2015). Dietary Lactobacillus culture did not effect on total aerobes, total anaerobes, Lactobacilli, and Streptococci in the small intestines and ceca of broiler chickens (Jin et al., 1998). However, diets with B. coagulans inclusion were significant improvement the Lactobacilli concentrations and tended to reduce Coliform in the duodenal digesta of broilers (Hung et al., 2012). Dietary B. licheniformis and B. subtilis significantly enhanced total Lactobacillus and Bifidobacterium sp. populations, and decreased E. coli and Salmonella sp. counts of cecal broilers (Yang et al., 2017). Supplemental probiotic mixture raised contents of both Lactobacillus and Bifidobacterium, and decreased coliform numbers in the small intestine of broilers (Song et al., 2014). Addition of B. subtilis in bird diets significantly enhanced Lactobacillus numbers in the cecum, ileal, and excreta, and declined E. coli counts in the cecum and excreta (Jeong and Kim, 2014).
Diets of BCP were no able to modulate the Lactobacilli proliferations and decline the E. coli numbers of ileal digesta of male broilers. The digestive tract of poultry consists of dynamic and interacting microbial ecosystems to maintain intestinal homeostatic (Pan and Yu, 2014). This status makes the host to keep the sustainability of microbiota balances by controlling indigenous contents of intestinal tract (Rehman et al., 2007). Brisbin et al. (2008) reported the gut-associated lymphoid tissue (GALT) of avian species is crucial protector for generating mucosal immune responses and maintaining microbiome homeostasis in the GIT.
The major causes of BCP failed in broiler diets for modulating Lactobacillus colonization and decreasing E. coli in the digestive tract of chickens are not certainly known. The interaction between the commensal bacteria, probiotics and the host must be tightly regulated, as host responses to members of the intestinal microbiome may contribute to the risk of inflammatory in mucosal tissues. An efficacy of probiotic supplementations depends on many factors such as bacterial species and viability, application methods, frequency of administrations, diet formulations, age of birds, and environmental factors (Patterson and Burkholder, 2003). One of the main benefits to the host from probiotics is largely altered the probiotic ability to competitively exclude pathogens for colonizing the intestine. There is achieved by organizing biofilms and mucosal barrier to the intestinal epithelium effectively blocking the sites from destructive bacteria (Brisbin et al., 2008). Uncontrolled immune activation for the response to the intestinal microbiota would contribute a risk of excessive inflammation and intestinal obstructive. Dietary supplementation with probiotics might develop for existing intestinal homeostasis and improve intestinal integrity in the chickens.
Broilers fed BCP had significantly lower cholesterol concentrations in the carcass. This observation was consistent with previous studies, birds fed B. subtilis (Santoso et al., 1995) had significantly reduced cholesterol levels in the carcass and liver. Supplementation of Lactobacillus culture in broiler diets dropped significantly serum cholesterol contents (Jin et al., 1998). Broilers administrated Lactobacillus culture were also reported in lowering significantly on serum total cholesterol, low density lipoprotein, and triglycerides of broilers at day 21 to 40 of age (Kalavathy et al., 2003). In contrast, the supplementations of B. coagulans ATCC 7050 were no significant differences in serum cholesterol, triglycerides, high density lipoprotein and low density lipoprotein concentrations (Hung et al., 2012). The mechanisms of cholesterol reduction by probiotics were unclear in this study. Probiotics are able to deconjugate bile salts by bile salt hydrolase enzyme, contributing in large excretion of bile acids in excreta (Klaver and Van Der Meer, 1993). Reis et al. (2017) reported the potential mechanisms responsible for the hypocholesterolemic effect of regular feeding of probiotic bacteria include: bile salts deconjugation, modulated lipid metabolism, decreasing absorption of intestinal cholesterols via co-precipitation of intestinal cholesterol with the deconjugated bile salts, incorporation and assimilation of cholesterol in the cell membrane of the probiotics, and inhibited expression of the intestinal cholesterol transporter Niemann–Pick C1 like 1 (NPC1L1) in the enterocytes. Thus, it would be contributed to reduce cholesterol in the carcass of broilers.
The number of previous studies has suggested that probiotics can enhance nutrient uptake via histomorphometric changes of villi in the small intestine, and humoral immunity modulations and intestinal flora alterations (Awad et al., 2009; Zhang and Kim, 2014). The Ileum fragment of the small intestine was responsible to absorb amino acids and minerals quickly (Leeson and Summer, 2005). The improvement in the villus height and crypt depth was associated with increasing of growth performance for probiotic (Awad et al., 2009). In the other hand, broiler fed B. Amyloliquefaciens improved apparent total tract nutrient digestibility of dry matter, crude protein, and apparent metabolizable energy (Lei et al., 2014). Manafi et al. (2016) presented that broilers fed B. subtilis markedly increased the digestibility of crude protein, crude fiber and gross energy. Similar positive influences were also reported by other researchers (Jeong and Kim, 2014).
In this observation, BCP treatment groups improved significantly the TME and TMEn of male birds. Dietary probiotics have been suggested for generating a better micro ecosytem of the gut by blocking microbial pathogen, increased beneficial microbe colonization, and modulated histomorphological of small intestine (Awad et al., 2009), and improved intestinal enzyme activity (Wang and Gu, 2010) that could rise healthy status of absorption organ. Therefore, there improved mucosal barrier function of the gut (Song et al., 2014), enhanced immunity (Xu et al., 2017), and enlarged surface area of nutrient uptake (Hung et al., 2012). It could promote the efficiency of nutrient digestibility, utilization, and retention in generating markedly metabolizable energy of broiler chickens. The probiotics may alter the synthesis and catabolism of amino acids in the small intestine, making changes of amino acids in the ileal digesta (Wu, 1998). An increased net energy for production requirement and body maintenance was satisfied; energy can be stored as a body fat (Wang et al., 2017). A net energy number applied for higher productivity correlated to the broiler performance and was an optimal energy utilization response. The fact that supplementation with105 CFU/g of BCP promotes broiler performance more effectively compared to the other levels that highlight the significance of the probiotic administration levels. This indicates that the probiotic can be used as a growth promoter in chicken diets and gut health modification.
CONCLUSION
Dietary supplementation with 105 CFU/g of B. coagulans D3372 could improve the growth performance, European production efficiency factor, and reduce cholesterol levels of carcass in broilers. Regarding this research, probiotics could enhance protein retention, fat retention, and metabolizable energy utilization, without affecting the ileal microbes of broilers.
ACKNOWLEDGMENTS
The authors would like to express our gratefulness to Kyushu Medical Co. Ltd., for funding and collaboration with Animal Science Faculty, Bogor Agricultural University.
CONFLICT OF INTERESTS
The author declares that there is no conflict of interest.
authors contribution
All authors were actively involved from designing, commencement, and data analysis of the experiment as well as wrting the manuscript.
REFERENCES






