Advances in Animal and Veterinary Sciences
Research Article
Clove Oil as Anesthetic for Tambacu Juveniles (♂Piaractus mesopotamicus × ♀ Colossoma macropomum)
William Franco Carneiro1, Naiara Melo1, Kiara Cândido Duarte da Silva2, Isabela Simas Ferreira1, Daniella Aparecida de Jesus Paula2, Marcos Ferrante1, Luis David Solis Murgas1,2*
1Department of Animal Science, Federal University of Lavras, Lavras, Minas Gerais, Brazil; 2Department of Veterinary Medicine, Federal University of Lavras, Lavras, Minas Gerais, Brazil.
Abstract | The objective of this study was to determine the clove oil (CLO) dose as an anesthetic for tambacu juveniles (♂Piaractus mesopotamicus × ♀ Colossoma macropomum) at two temperatures. Sixty-four animals (2.42 ± 0.39 g and 5.19 ± 7.5 cm) were used in a 4x2 factorial design, composed by four concentrations of CLO (25, 50, 75 and 100 mg L-1) and two temperatures (25 and 30 °C). Data were submitted to a two-way ANOVA followed by Tukey test. Linear regression analysis was also applied to correlate the induction and recovery times of fish. Deep anesthesia was achieved at all concentrations tested and no mortality was observed during the experimental procedure and after a 24-hour observation period. The shortest time for deep anesthesia (31.75 s) was observed at a concentration of 100 mg L-1 and a temperature of 30 °C, differing significantly from the other treatments. The longest recovery time (> 270 s) was observed at concentrations of 75 and 100 mg L-1 at both temperatures tested. A positive linear relationship between induction time and deep anesthesia (P<0.05) and a negative linear relationship between total recovery time (P<0.05) was observed. The CLO was effective to anesthetize tambacu juveniles in routine management activities. We recommend to use the 50 mg L-1 concentration of CLO and a temperature of 30 °C when considering the induction and recovery times for a deep anesthesia.
Keywords | Aquaculture, Neotropical fish, Management, Eugenol, Recovery
Received | June 25, 2019; Accepted | August 19, 2019; Published | October 25, 2019
*Correspondence | Luis David Solis Murgas, Department of Animal Science, Federal University of Lavras, Lavras, Minas Gerais, Brazil; Email: [email protected]
Citation | Carneiro WF, Melo N, Da Silva KCD, Ferreira IS, Paula DAJ, Ferrante M, Murgas LDS (2019). Clove oil as anesthetic for tambacu juveniles (♂piaractus mesopotamicus × ♀ colossoma macropomum). Adv. Anim. Vet. Sci. 7(11): 969-976.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.11.969.976
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Murgas et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The use of anesthetics is very common in fish capture, handling and transport activities. Anesthetics are known to be effective and to minimize the deleterious effects of stress caused by fish management (Zahl et al., 2012). The choice of anesthetic is usually related to several factors, such as availability, economic viability, practicality of use, efficacy, user safety and chemicals substances approved by regulatory agencies in animals for human consumption (Cho and Heath, 2000; Gimbo et al., 2008; Gonçalves et al., 2008).
A variety of anesthetic agents are widely used in laboratories, veterinary procedures and aquaculture activities. The most commonly used anesthetics in fishes are MS-222 (Tricaine), benzocaine, metomidate, 2-phenoxyethanol and quinaldin (Neiffer and Stamper, 2009; Ross and Ross, 2008; Stringhetta et al., 2017). These anesthetics were evaluated for induction, recovery and pharmacokinetic times (Sneddon, 2012).
However, much of this research is limited to a restricted number of species, so care should be taken when using any of these agents on unvalidated species, as the reaction, tolerance and anesthetic type may differ between species (Sneddon, 2012; Zahl et al., 2012). Although widely used, many of these anesthetics have been associated with undesirable systemic side effects and restricted safety margins (Palić et al., 2006; Cunha et al., 2010). On the other hand, in recent years essential oils extracted from plants have been used as alternative anesthetic agents for fishes. Some of the essential oils studied were obtained from Lippia alba (Cunha et al., 2010; Toni et al., 2014; Souza et al., 2018), Ocimum gratissimum (Boijink et al., 2016; Ribeiro et al., 2016), Cinnamomum camphora (Pedrazzani and Neto, 2016), Mentha piperita (Mazandarani and Hoseini, 2017) and Syzygium aromaticum (Javahery et al., 2012).
Clove oil (CLO) is a product resulting from distillation of Syzygium aromaticum leaves, stem, flowers and buds, consisting mainly of eugenol (4-allyl-2-methoxyphenol) and isoeugenol (4-propenyl-2-methoxyphenol) that comprise 90-95% of the oil (Javahery et al., 2012). CLO has shown to be a potential and effective anesthetic for some fish species, such as medaka (Oryzias latipes), goldfish (Carassius auratus), carp (Cyprinus carpio), channel catfish (Ictalurus punctatus), Atlantic salmon (Salmo salar) and pacu (Piaractus mesopotamicus) (Woody et al., 2002; Endo et al., 1972; Hikasa et al., 1986; Waterstrat, 1999; Chanseau, 2002; Rotili et al., 2012). In addition to efficiency, some studies reported that CLO is less toxic and leaves no residue in fish meat (Sladky et al., 2001, Soto and Burhanuddin, 1995).
Tambacu is a neotropical hybrid fish resulting from reproduction between pacu (Piaractus mesopotamicus) males and tambaqui (Colossoma macropomum) females. This hybrid has important characteristics for production such as robustness, high fecundity and rapid growth rate (Martins et al., 2002; Gonçalves et al., 2010). However, there are few studies using anesthetics in tambacu (Sena et al., 2016; Limma-Netto et al., 2018).
Considering the above-mentioned facts and limited information about the use of CLO in tambacu, the present study aimed to evaluate the efficacy of different concentrations of CLO, at two temperatures (25 and 30 °C), in anesthetic induction and recovery times of tambacu juveniles.
MATERIAL AND METHODS
Test Animals and Acclimatization Conditions
This research was conducted at the Central Bioterium of the Federal University of Lavras (UFLA), Minas Gerais, Brazil. Sixty-four tambacu (♂ Piaractus mesopotamicus × ♀ Colossoma macropomum) juveniles, obtained from a commercial fish farming, were used for the experimental realization, with an average weight and length of 2.42 ± 0.39 g and 5.19 ± 7.5 cm, respectively. Prior to the experimental period, the animals were acclimatized in a 500 L polyethylene tank of usable volume in a water recirculation system. The animals were kept under a photoperiod of 12h light/12h dark.
The fish were fed extruded commercial feed containing 45% crude protein and particle size of 1.3 mm, supplied three times a day until apparent satiety. After the last feeding, tank was siphoned daily for removal of organic matter. Water quality parameters such as pH, total ammonia and nitrite were monitored daily by colorimetric kits. Temperature and dissolved oxygen were measured daily with oximeter (Alfakit model AT 150). After the acclimatization period, the animals were fasted for 24 hours and subsequently distributed into the experimental aquariums. All procedures performed in this study were in accordance with the ethical procedures advocated by Council on Animal Experimentation of Federal University of Lavras.
Anesthetic Solution
The concentrations of CLO (Asfer Indústria Química LTDA, Brazil) used in the present study (85-95% eugenol) were expressed in mg L-1, because the density of clove oil is approximately 1 g mL-1 (Soto and Burhanuddin, 1995). To ensure the uniformity of CLO, the concentrations were first dissolved in 1:10 ethanol and then stored in an amber stock vial until its use.
Experimental Design
The experiment was conducted in a 4x2 factorial design with four concentrations of CLO (25, 50, 75 and 100 mg L-1), two temperatures (25 and 30 °C) and eight replicates. The treatments were randomly distributed into 30 L glass aquariums (total volume), filled with 20 L water. For all aquariums a constant aeration system was provided by porous stones connected to a silicone hose through 9-watt electromagnetic air pump (Boyu S4000B, Guangdong, China). Water temperature was controlled with 50-watt adjustable heating pipe in each experimental unit (Roxin HT-1300).
The water quality parameters were maintained under the same conditions of the system in which the fishes were acclimated, and were always measured before the beginning of the experimental procedures. The mean values observed for water quality parameters were within those described as optimal for the tambacu (Baldisseroto 2002; Gonçalves et al. 2010).
Different anesthetic concentrations and water for recovery of the animals were renewed at each procedure to avoid variation in anesthetic concentrations and metabolite accumulation in the recovery aquarium. During the experimental procedure, the times for each stage of anesthesia and recovery were measured (Table 1) and, after reaching anesthesia stage A3, fishes were removed from the aquariums, weighed, measured and transferred to the recovery aquariums (without anesthetic), and the recovery time of each animal was monitored until they reach stage R3. Subsequently, the animals were transferred to 500 L tanks, wh-
Table 1: Stages of anesthetic induction and recovery in clove oil efficacy tests in tambacu juveniles (♂Piaractus mesopotamicus x ♀Colossoma macropomum).
| Stage | Description | Observed behavior |
|
Anesthesia inductiona |
||
| A1 | Light sedation | Partial loss of muscle tone, erratic swimming |
| A2 | Light anesthesia | Complete loss of balance, normal or slow ventilation |
| A3 | Deep anesthesia | Total loss of muscle tone and no reaction to handling |
|
Recoveryb |
||
| R1 | Initial recovery | Normalization of opercular movements |
| R2 | Partial recovery | Return of swimming in the water column |
| R3 | Full recovery | Responses to tactile stimulation |
aInduction stages modified by Ross and Ross, 2008; Gressler et al., 2017.
bRecovery stages modified by Braz et al., 2017.
ere they remained under observation for 24 h to assess survival.
Statistical Analysis
Anesthesia and recovery time data at different temperatures were submitted to Analysis of Variance (Two-way ANOVA) to test the significance of the effects of temperature and anesthetic concentration on clove oil efficacy. When significant differences were found, Tukey’s multiple comparisons test was applied. A significance level of 5% (P<0.05) was considered for all analyzes (Bhujel, 2008). Regression equations were used to explain the relationship between anesthetic concentrations and induction and recovery times. All data were analyzed using Minitab 17 statistical package (Minitab LLC, State College, PA, USA)).
RESULTS
No mortality was observed in any of the concentrations during anesthesia procedures, manipulation and 24 h after exposure. The effects of different concentrations of CLO on each temperature are shown in Table 2. The time for anesthetic induction was significantly (P<0.05) affected by temperature and concentrations of clove oil. We observed that the increase in concentrations of clove oil and temperature linearly decreased the time for deep anesthetic induction. At each temperature, the time required for the anesthetic to take effect decreased significantly as concentration of clove oil increased (P<0.05). At each concentration of clove oil the time required to anesthetize animals decreased significantly with increasing temperature (P<0.05).
We observed that at 25 °C the time to reach light sedation stage was significantly (P<0.05) longer at 25 mg L-1 dose. With an increase to 100 mg L-1, at both temperatures, the time for light sedation decreased (P<0.05). The time for deep anesthetic induction at a clove oil concentration of 100 mg L-1 at 30 °C (31.75 ± 2.66 s) was significantly shorter when compared to other doses. On the other hand, the dose of 25 mg L-1 clove oil at 25 °C resulted in the longest time for deep anesthetic induction. However, there was no significant difference between the doses of 25 mg L-1 at 30 °C and 50 mg L-1 at 25 °C.
The time required to restore fish balance after anesthetic induction with clove oil was significantly (P<0.05) different for the concentrations and temperatures tested (Table 3). Fishes exposed to doses of 75 and 100 mg L−1 presented significantly longer total recovery time (P<0.05) than animals exposed to other concentrations. Animals submitted to 50 mg L−1 clove oil at 30 °C showed total recovery similar to animals receiving a 25 mg L−1 dose at both temperatures.
When concentrations of CLO were evaluated, regardless of water temperature, we observed that the time taken by animals to enter the stage A1 at doses of 100 and 75 mg L-1 was significantly (p<0.05) shorter (< 20 s) than other doses. The dose of 50 mg L-1 presented intermediate values and the lowest dose tested was the one with the longest time (> 38 s) for fish reaching light sedation stage (Figure 1A).
We observed that the time taken for the animals reaching A2 stage at doses of 75 and 100 mg L-1 was significantly (P<0.05) shorter than other concentrations. The longest time (> 70 s) to reach light anesthesia stage was observed in tambacu juveniles exposed to dose of 25 mg L-1 (Figure 1B). Significant differences (P<0.05) were found between the highest concentrations (75 and 100 mg L-1) and the lowest (50 and 25 mg L-1) of clove oil when compared to the time took by fishes to reach deep anesthesia stage (Figure 1C).
No significant differences (P>0.05) were observed in the initial recovery (R1) of fishes in any of the tested doses. The average time observed for R1 was between 14 and 23 s
Table 2: Induction time for tambacu (♂Piaractus mesopotamicus × ♀ Colossoma macropomum) anesthetized with clove oil at different temperatures (25 and 30 °C).
|
Dose (mg L−1) |
Temperature (°C) | Anesthetic induction time at each stage* | |||
| A1 | A2 | A3 | |||
| 25 | 25 | 40.33±3.90ª | 99.88±8.46ª | 236.88±17.37ª | |
| 25 | 30 |
26.50±4.44bc |
45.88±8.84bc |
126.13±18.11b |
|
| 50 | 25 |
32.25±2.20ab |
51.43±3.78b |
124.50±13.59b |
|
| 50 | 30 |
19.88±2.34cd |
43.63±1.65bc |
94.00±17.90bc |
|
| 75 | 25 |
17.75±1.47cd |
29.71±1.49bcd |
73.57±9.05bcd |
|
| 75 | 30 |
16.25±1.63cd |
30.00±2.16bcd |
45.38±6.25cd |
|
| 100 | 25 |
13.75±1.11d |
25.25±1.06cd |
57.00±4.95cd |
|
| 100 | 30 |
11.63±0.50d |
21.88±0.55d |
31.75±2.66d |
|
|
Dose (mg L−1) |
|||||
| 25 | 38.31±5.20 | 72.88±9.14 | 181.50±18.74 | ||
| 50 | 26.06±2.23 | 54.00±7.03 | 109.25±11.55 | ||
| 75 | 17.00±1.08 | 31.19±1.80 | 64.38±8.36 | ||
| 100 | 12.56±0.68 | 23.56±0.72 | 44.38±4.24 | ||
| Temperature (°C) | |||||
| 25 | 28.47±3.20 | 55.47±6.65 | 125.44±13.78 | ||
| 30 | 18.50±1.60 | 35.34±2.83 | 74.31±9.24 | ||
| Two-way ANOVA (p-value) | |||||
| Dose | <0.001 | <0.001 | <0.001 | ||
| Dose x temperature | 0.002 | <0.001 |
0.003 |
||
* Each value represents the average ± SEM (n = 8).
Values in each column that do not share a common superscript are significantly different from one another (P<0.05).
Table 3: Recovery time for tambacu (♂Piaractus mesopotamicus × ♀ Colossoma macropomum), anesthetized with clove oil at different temperatures (25 and 30 °C).
|
Dose (mg L−1) |
Temperature (°C) | Recovery time (s) at each stage* | ||
| R1 | R2 | R3 | ||
| 25 | 25 |
34.75±5.76a |
94.25±15.48cde |
196.25±11.12b |
| 25 | 30 |
9.71±1.70b |
69.25±5.57e |
174.13±17.69b |
| 50 | 25 |
24.13±44.59ab |
130.75±6.63bc |
290.00±13.11a |
| 50 | 30 |
14.75±3.55b |
72.88±9.56de |
187.75±13.82b |
| 75 | 25 |
13.43±1.41b |
119.00±9.89bcd |
292.13±8.74a |
| 75 | 30 |
15.63±0.65b |
119.75±7.47bc |
279.71±13.73a |
| 100 | 25 |
13.25±1.37b |
189.86±11.46a |
292.71±26.56a |
| 100 | 30 |
15.63±1.86b |
161.38±13.80ab |
280.50±9.40a |
|
Dose (mg L−1) |
||||
| 25 | 23.07±4.54 | 81.75±8.58 | 185.19±10.49 | |
| 50 | 19.44±3.05 | 101.81±9.35 | 238.88±16.09 | |
| 75 | 15.44±1.11 | 125.44±8.16 | 285.63±8.04 | |
| 100 | 14.44±1.16 | 185.25±13.86 | 297.56±14.30 | |
| Temperature (°C) | ||||
| 25 | 21.84±2.40 | 141.31±10.57 | 273.25±11.29 | |
| 30 | 14.06±1.15 | 105.81±8.17 | 230.38±11.12 | |
| Two-way ANOVA (p-value) | ||||
| Dose | 0.091 | <0.001 | <0.001 | |
| Dose x temperature | <0.001 | 0.061 |
0.003 |
|
* Each value represents the average ± SEM (n = 16).
Values in each column that do not share a common superscript are significantly different from one another (P< 0.05).
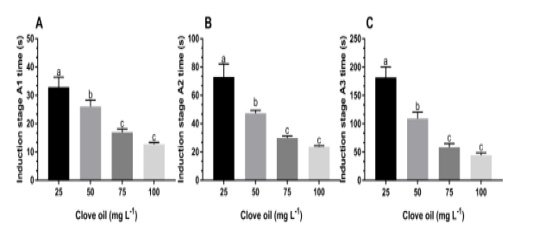
Figure 1: Time (s) to reach light sedation (A), light anesthesia (B) and deep anesthesia (C) stages at different concentrations of clove oil for tambacu juveniles (♂Piaractus mesopotamicus x ♀Colossoma macropomum). Values (mean ± SEM, n = 16) with different letters are significantly different between anesthetic concentrations.
after fishes were allocated into recovery tanks. On the other hand, the time for partial (R2) and total (R3) recovery showed significant differences (P<0.05) between concentrations of clove oil (Figure 2).
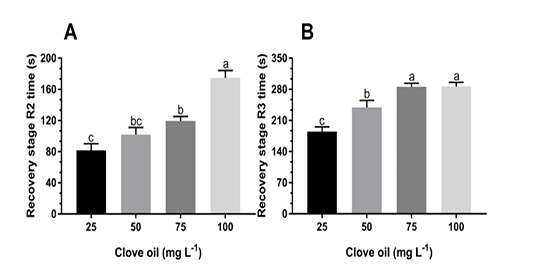
Figure 2: Partial recovery time (s) R2 (A) and total recovery R3 (B) at different concentrations of clove oil for tambacu juveniles (♂Piaractus mesopotamicus x ♀Colossoma macropomum). Values (mean ± SEM, n = 16) with different letters are significantly different between anesthetic agent concentrations.
The shortest partial recovery times were observed in juveniles submitted to 25 mg L-1 (81.75 ± 8.58 s). The concentration of 100 mg L-1 clove oil resulted in the longest time (185.25±13.86 s) for partial recovery of animals, differing significantly (P<0.05) from other doses. The doses of 75 and 100 mg L-1 clove oil presented the highest times for total recovery, with an average of 285.63 ± 8.04 s and 297.56±14.30 s, respectively. On the other hand, the lowest evaluated concentration presented the shortest total recovery times of tambacu juveniles (185.19 ± 10.49 s), whereas the anesthetized animals with 50 mg L-1 showed intermediate total recovery times (P<0.05), with an average of 238.88 ± 16.09 s.
A significant relationship was observed between anesthetic concentration and deep anesthetic induction time (A3) for clove oil (P <0.05). Total recovery time (R3) showed a significant linear relationship (P <0.05) inversely proportional to the total time for induction of deep anesthesia (Figure 3).
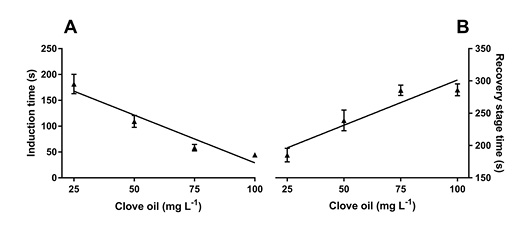
Figure 3: Relationship between deep anesthetic induction time (s) (A) and total recovery time (B) at different concentrations of clove oil for tambacu juveniles (♂Piaractus mesopotamicus x ♀Colossoma macropomum). Values are presented as mean ± standard error of the mean (SEM) (n = 16).
DISCUSSION
Anesthetics are an essential component in aquaculture activities, as their main function is to facilitate handling of animals and minimize their stress. Anesthetics are widely used in fish management because they allow researchers and professionals to measure, weigh, mark individuals, apply antibiotics, vaccines and draw blood from fish (Serezli et al., 2012; Wilson, 2005). However, as stress responses vary widely between species it became necessary to find the appropriate dose for each cultivated species (Pawar et al., 2011; Ross and Ross, 2008). In the tested concentrations, the present study demonstrates that CLO acts as anesthetic in tambacu juveniles and can be used in aquaculture applications without causing damage to animals. Other studies report the efficacy of CLO as an anesthetic in Paracheirodon axelrodi, Heros severus, Pterophyllum scalare and Danio rerio (Fujimoto et al., 2018; Grush et al., 2004).
Some authors suggest that the criteria for determining anesthetic efficacy include: (i) anesthesia in 3 min, (ii) recovery in 10 min and (iii) without mortality during the period that anesthetic agents have been used (Ross and Ross, 2008). It is noteworthy that the use of lower anesthetic concentrations should be considered in order to provide a greater margin of safety for animals, avoid unnecessary expenses and waste of anesthetic solution (Teixeira et al., 2017).
In the present study we observed that concentrations of 25 to 100 mg L-1 CLO, at two different temperatures (25 and 30 °C), resulted in anesthetic induction in less than 3 min. This except for 25 mg L-1 dose at 25 °C which took approximately 4 min, twice the time observed at the same dose at 30 °C. These results prove the efficacy of clove oil even at low doses (25 mg L-1), when taking into account temperature control at the time of anesthetic induction.
These results demonstrate that in addition to the species to be anesthetized, life stage, size and weight, temperature is among the factors that affect metabolic rate and consequently, drug pharmacokinetics (Iversen et al., 2003; Snedon et al., 2012; Gressler et al., 2017). Similar effect was observed in Oryzias latipes, where a significant decrease in anesthetic induction times with increasing water temperature by 5 °C (Endo et al., 1972).
The variation in fish body temperature affects the speed of chemical reactions, as its raises the kinetic energy of atoms and molecules (Baldisserotto, 2002). On the other hand, the increase in fish body temperature raises the metabolic rate and consequently, the oxygen consumption (Schmidt-Nielsen, 2002). The high oxygen demand of fish can be compensated by increased breathing movements, allowing a larger volume of water to pass through the gills as well as dissolved substances (Schmidt-Nielsen, 2002). This facilitates rapid absorption and anesthetic distribution as well as faster clearance rates (Zahl et al., 2012). However, some precautions should be taken into consideration since a greater amount of anesthetic agent may be absorbed.
The negative linear effect observed in total anesthetic induction time was similar to that reported by Pereira-da-Silva et al. (2009), which evaluated different concentrations of clove oil for lambari (Astyanax altipanae) fingerlings. On the other hand, in dourado (Salminus brasiliensis) juveniles submitted to different concentrations of clove oil (20, 30, 40, 50 and 60 mg L-1) a polynomial effect was reported by Hisano et al. (2008) on anesthetic induction times.
A huge decrease in anesthetic induction time at initial concentrations, with a tendency to stabilization in the highest concentrations of clove oil, was observed in Oncorhynchus nerka, Leporinus macrocephalus and Oreochromis niloticus (Woody et al., 2002; Vidal et al., 2007; Vidal et al., 2008). Many differences in reaction to anesthesia between species, size, environmental conditions in which fishes are found, besides the type of anesthetic agent used, are found in the literature, thus requiring that all factors should be studied for the species of interest.
Although total recovery time of fishes is less crucial in a field situation, a rapid and complete resumption of normal physiological activities is desirable (Walsh and Pease, 2002). In this study, we observed that the time for full recovery of animals increased progressively with increasing clove oil concentrations. The results observed in this study are in agreement with previous works (Pawar et al., 2011; Gullian and Villanueva, 2009, Weber et al., 2009, Heo and Shin, 2010). The authors suggested that the recovery time decreases inversely with the anesthetic agent concentration in teleosts. Despite increased concentrations of clove oil resulted in a longer time for recovery of tambacus, we observed that 75 and 100 mg L-1 doses did not differ from each other, regardless of the temperature evaluated, as well as the concentration of 50 mg L-1 at temperature of 25 °C. Regardless of concentrations of 75 and 100 mg L-1 show the longest times (<5 min) for total recovery of fishes, these results are within the time limit advocated by Marking and Meyer (1985) for total recovery of fishes.
The use of eugenol as an anesthetic for fishes has its cost as a positive factor, since the price per dose is approximately 10 times lower when compared to a similar dose of MS-222 (Roubach et al., 2005). However, further research should focus on efficacy of clove oil in other species, as well as elucidating the mechanisms of action, metabolization, excretion and possible residual effects on fishes (Pádua et al., 2018). According to Roubach et al. (2005), with this information, the agencies responsible for regulating the use of chemicals in animals for human consumption could support the use of clove oil as an anesthetic in fishes.
The CLO proved to be a safe and efficient anesthetic for tambacu juveniles, since none of the doses tested showed mortality. Also, recovery of the animals occurred outside the critical range (Marking and Meyer, 1985). Considering the appropriate time for deep anesthesia all concentrations tested are sufficient for tambacu juveniles, except the concentration of 25 mg L-1 at 25 °C. To increase safety during anesthetic and recovery procedures the concentrations of 25 mg L-1 at 30 °C or even 50 mg L-1 at 25 °C can be used.
In conclusion, the concentration of 50 mg L-1 and water temperature at 30 °C proved to be efficient for deep anesthesia in tambacu juveniles in routine management activities. Moreover, it ensures the shortest times for full recovery of fishes.
ACKNOWLEDGEMENTS
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Nível Superior (CAPES), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Universidade Federal de Lavras (UFLA).
CONFLICT OF INTEREST
All authors declare there is no conflict of interest.
AUTHORS CONTRIBUTION
WFC, NM, KCDS, ISF and LSDM: conceived the original idea. WFC, NM, KCDS, ISF and LDSM: planning and execution of this work. WFC, NM, DAJP, MF and LDSM: data analysis. WFC, DAJP, MF and LDSM: wrote the manuscript. All authors read and approved the final manuscript.
REFERENCES






