Advances in Animal and Veterinary Sciences
Research Article
Modulatory Effect of Ginger Aqueous Extract on Imidacloprid Induced Hepatotoxicity in Rats
Mayada R. Farag, Magdy F. Abou-EL Fotoh, Gihan G. EL-Sayed, Eman W. EL-Sayed*
Forensic Medicine and Toxicology Department, Veterinary Medicine Faculty, Zagazig University, Zagazig, Egypt.
Abstract | The current study was conducted for evaluation of the hepatotoxic effect of Imidacloprid (IMI) in rats and to assess the modulatory role of Zingiber officinale Roscoe, ginger aqueous extract (GAE), against such effect. Sixty mature male rats (Rattus norvegicus) were utilized and divided into six groups (10 each); group 1 was negative control received 0.1 ml of distilled water, group 2 was positive control which received 1ml of GAE, group 3 administered with 0.1 ml of IMI, group 4:administered 1ml of GAE for 2 weeks followed by administration of 0.1ml of IMI (served as protected group), group 5 administered with 0.1 ml of IMI then 1ml of GAE for 2 weeks (served as treated group) and the last group simultaneously administered with 0.1ml of IMI and 1ml of GAE (served as combination group). Oral dosing of IMI and GAE was triple weekly (day after day) and the experimental period was three months for all groups. IMI exposure caused significant increase in alanine aminotransferase (ALT), aspartate amino transferase (AST) levels, significant decrease in total proteins, albumin and globulins in serum, IMI upregulates interleukin 6 (IL6), tumor necrosis factor- α (TNF-α) and toll like receptor 2 (TLR2) genes in the liver of rats associated with intense immuno positive reactivity of TLR2 and TNF α in the liver of IMI-treated group. Histopathological examination revealed significant alterations in the liver tissue of IMI-treated group, such as disorganization of hepatic cords replaced by mononuclear cells which surrounded by lymphocytes and mild congestion of blood vessels was also seen. In conclusion, the IMI hepatotoxic effect could be modulated by the use of GAE.
Keywords | Imidacloprid, Ginger, TLR2, TNFα
Received | September 11, 2019; Accepted | October 06, 2019; Published | October 10, 2019
*Correspondence | Eman W. EL-Sayed, Forensic Medicine and Toxicology Department, Veterinary Medicine Faculty, Zagazig University, Zagazig, Egypt; Email: [email protected]
Citation | Farag MR, Fotoh MFA, EL-Sayed GG, EL-Sayed EW (2019). Modulatory effect of ginger aqueous extract on imidacloprid induced hepatotoxicity in rats. Adv. Anim. Vet. Sci. 7(s2): 34-43.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s2.34.43
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Farag et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Inappropriate and indistinctive usage of insecticides may result in their deposits in the food chain that may exert their detrimental effects in target and non-target organisms (Geiger et al., 2010; Lopez-Antia et al., 2013). Neonicotinoid pesticides are synthetic derivatives of nicotine, an alkaloid compound originated in the leaves of various plants as well as tobacco (Costa, 2008). Neonicotinoid insecticides act specifically as agonists on nicotinic acetylcholine receptors (nAChRs) of pests, (Jeschke et al., 2010), competing for these receptors on postsynaptic membrane (Tomizawa and Casida, 2005; Anderson et al., 2015). Neonicotinoid is one of the most sold pesticides internationally nowadays. These pesticides are used as leaf spray, seed coating, and soil drenches when used in harvests (Bonmatin et al., 2015). In the last decade, there are endless fears about the injurious effects of IMI, neonicotinoid insecticide, and its degradation byproducts on the atmosphere and finally public health (Mohamed et al., 2017). IMI is frequently applied on cereals, maize, rice, vegetables, potatoes, fruits, cotton, sugar beets, and turf and can be utilized for the treatment of soil or seed. IMI has gained the most sold pesticide worldwide for horticultural use and for veterinary therapy to control external parasites in the past decade (Badgujar et al., 2013). Hepatic impairment occurred mostly due to exposure to toxic chemicals, environmental pollutants for the last few decades (Atta et al., 2010). The hepatotoxicity of neonicotinoids is measured by evaluating the enzymatic activity and liver histopathological changes, which were degeneration of hepatocytes, central vein dilations, and elevation of liver enzymes (Toor et al., 2013; Vohra et al., 2014; Kapoor et al., 2014). Ginger, the rhizome of Zingiber officinale Roscoe (a member belonging to the Zingiberaceae family) are commonly used as a spice or dietary supplement with a long history of utilization in the traditional medicine (Yusof, 2016). About 400 kinds of ingredients have been identified in the ginger, however, the pharmacological effect of ginger is largely attributed to the gingerols, shogaols, zingerone and paradols (Prasad and Tyagi, 2015). 6-Gingerol is the major pungent compound in fresh ginger with various biological properties and it has been connected to ameliorating or preventing chronic diseases in human and animal models (Wang et al., 2014; Yusof and Anum, 2016). Ginger has been reported to exhibit hepatoprotective action via its antioxidant potential (Liu et al., 2013).
This study aimed to investigate the hepatotoxic impact of IMI via estimation the levels of ALT, AST, TP, albumin, globulin, gene expression of TLR2, TNF α and IL6 in the liver tissue of rats, along with histopathological examination and immunohistochemical staining of TLR2, TNFα in liver.
MATERIAL AND METHODS
Tested compounds
imidacloprid (IMI) (C9H10ClN5O2, CAS No. 138261-41-3) was purchased as cloprid plus 35% SC produced by European Community for agricultural products, Germany. IMI was dissolved in distilled water and orally administered to rats throughout the experimental period.
Ginger aqueous extract; ginger (Zingiber officinale Roscoe) rhizomes were obtained from the local market at AL-Sharkia province, Egypt. One kilogram of the ginger rhizomes was cleaned, washed under faucet water, at that point cut into little portions, air dried and powdered. 125 g of the powder were soaked in 1000 ml of distilled water for 12 h at room temperature and were then sieved. The concentration of the extract is 24 mg/ml. Every rat in the current study was given 1 ml of the absolute aqueous extract orally (Kamtchouing et al., 2002).
Animals and experimental design
Sixty mature male rats (Rattus norvegicus) weighing (150-200gm) were obtained from the Laboratory Animal Housing Unit, faculty of Veterinary Medicine, Zagazig University, Egypt. The animals were clinically healthy, kept under hygienic conditions in hard wood shaving–bedded plastic cages. They were maintained on balanced ration and tap water ad-libtum and a photoperiod of 12/12 hrs light/dark cycle. Rats were kept for two weeks before starting the experiment for acclimatization, then randomly divided into six groups (10 rats each) as follow; group 1 was negative control that received 0.1 ml of distilled water, group 2 was positive control thatreceived 1ml of GAE, group 3 incoualted with 0.1 ml of IMI, group 4 was administered with 1ml of GAE for 2 weeks followed by administration of 0.1ml of IMI (served as protected group), group 5 treated with 0.1 ml of IMI then 1ml of GAE for 2 weeks (served as treated group) and the last group; group 6 administered with 0.1ml of IMI and 1ml of GAE simultaneously (served as combination group). Oral dosing of IMI and GAE was given triple weekly at the same time (day after day) for three months for all groups, IMI was given orally using rat stomach tube. The dose of IMI was selected to be 1/100 LD50 =0.21 mg/kg b.w. (Mohany et al., 2011). The concentration of IMI in the commercial product (cloprid) was 35% and dosing volume for each rat was 0.1ml. Animal care and experimental protocols were approved by Ethics of Animal Use in Research Committee (EAURC), Faculty of Veterinary Medicine, Zagazig University, Egypt.
Blood sampling and tissue collection
At the end of the experiment (3 months), blood samples were collected from retro-orbital venous plexus for obtaining sera samples. Blood samples were collected into clean, dry test tubes then centrifuged at 3000 rpm for 10 minutes and the sera were collected and kept at -20º until analysis. Then, the animals were fasted overnight, euthanized by cervical dislocation. Specimens from liver were collected, and divided into two parts at which the first part was directly preserved in liquid nitrogen for gene expression analysis while the second part was preserved in 10% neutral buffered formalin solution for histopathological examination.
Serum biochemical parameters and Gene expression of IL6, TNF-α and TLR2 genes
Commercial kits for TP, albumin, ALT, AST estimation in serum were used (BioMed Diagnostics, Egypt).
RT-qPCR was utilized to evaluate the mRNA expression levels of IL6, TNF-α and TLR2 genes in the liver of rats using gene-specific primer pairs (Table 1) following PCR conditions as detailed in (Table 2) (Ye et al., 2012). RNeasy Mini Kit (Qiagen, Heidelberg, Germany) was used to extract total RNA which reverse transcribed using a QuantiTect Reverse Transcription kit (Qiagen, Heidelberg, Germany) following the manufacturer’s instructions. RT-qPCR was performed on a Rotor-Gene Q cycler (Qiagen, Germany)
Table 1: Forward and reverse primers sequence used in qPCR.
| Accession number | Product size (bp) | '5-3' Primer sequence | Primers | Gene |
| NM_012589.2 | 168 | TCCTACCCCAACTTCCAATGCTC | forward | IL6 |
| TTGGATGGTCTTGGTCCTTAGCC | reverse | |||
| NM_012675.3 | 217 | GCATGATCCGCGACGTGGAA | forward | TNFa |
| AGATCCATGCCGTTGGCCAG | reverse | |||
| NM_198769.2 | 120 | CGCTTCCTGA ACTTGTCC | forward | TLR2 |
| GGTTGTCACCTGCTTCCA | reverse | |||
| NM_031144 | 492 | CCTCTATGCCAACACAGTGC | forward |
β-actin |
| GTACTCCTGCTTGCTGATCC | reverse |
Table 2: The thermal cycler condition used during real time PCR.
| Cycles | Extension | Annealing | Denaturation | Initial denaturation |
| 40 | 72˚C/ 30 sec | 60-61˚C/ 30 sec | 95˚C/ 15 sec | 95˚C/ 10 min |
using QuantiTect SYBR Green PCR kits (Qiagen, Heidelberg, Germany). Relative fold changes in the expression of target genes were accomplished using the comparative 2−ΔΔCt (Ct: cycle threshold) method (Livak and Schmittgen 2001) with the β-actin gene as an internal control to normalize target gene expression levels.
Immunohistochemistry of TNF-α and TLR2
For IHC staining, avidin biotin peroxidase method was used. The sections used were fixed on charged slides, then de-paraffinized in 4 changes of fresh xylene, and re-hydrated in graded ethanol (100 %, 95 % and 70 %) then washed in PBS at pH 7.2 for 5 minutes. To prevent endogenous peroxidase action, the sections were dipped in 0.3% hydrogen peroxide in water then washed 3X with distilled water then PBS. The sections were washed in 10% normal rabbit serum (blocking reagent) in a humid chamber for 30 minutes to reduce non-specific binding of immunoglobulins. The sections were incubated with antisera containing the specific primary antibody (Anti-TNF-α antibody, Anti-TLR2 antibody) and in a humid chamber overnight at room temperature. Excess reagents were thrown off and the slides were washed 2X with of PBS 5 minutes each. Then, the sections were covered with biotinylated secondary anti-immunoglobulin and incubated in a humid chamber at room temperature for 30 minutes then washed 2X with of PBS 5 minutes each. Labeling antibody (streptavidin enzyme label) was added to each section andkept in a humidified chamber at room temperature for 30 minutes then washed 2X with of PBS 5 minutes each. Diaminobenzidine (DAB) was used as chromogen and sections were incubated for 2-4 minutes at room temperature and the sections were washed in distilled water for 5 minutes then, were counter stained with Mayer’s haematoxylin, dehydrated in ascending grades of alcohol, cleared in xylene and mounted in Canda Balsam. Slides are visualized under a light microscope (Bancroft and Gamble, 2008).
Histopathological examination
Specimens from the liver were collected and fixed in 10% buffered neutral formalin solution, dehydrated in graded ethanol (70-100%), cleared in xylene, and implanted in paraffin. Five-micron thick paraffin sections were prepared and then regularly stained with hematoxylin and eosin (HE) dyes (Suvarna et al., 2013) and then examined using standard light microscope.
Statistical analysis
Data were analyzed using one way-ANOVA procedure. Duncan’s Multiple Range test was conducted to compare means between groups. Data were expressed as mean±SEM. A value of p < 0.05 was considered as statistically significant.
Table 3: Effects of IMI, GAE and their co-exposure on body weights (g)
| Item | Initial body weight | Final body weight | body weight change |
| Control | 182.80±1.53 |
244.01±12.32ab |
61.20±12.87ab |
| GAE | 181.30±1.72 |
267.64±9.34a |
86.33±10.99a |
| IMI | 180.36±4.63 |
202.37±2.93c |
22.01±6.65c |
| GAE/IMI | 181.21±0.63 |
228.33±9.17bc |
47.11±9.22bc |
| IMI/GAE | 181.60±2.62 |
217.33±2.72bc |
35.73±3.92bc |
| IMI+GAE | 182.11±2.83 |
246.00±2.64ab |
63.89±4.63ab |
| Sig. | NS | ** | ** |
Means within each column carrying different superscript are significant at (P ≤ 0.01); Star value: ** means significant at (P ≤ 0.01).
RESULTS
Signs of intoxication
Signs of intoxication were mainly decreasing of feeding behavior in IMI- intoxicated rats accompanied with decreased body weight while there was no change in
Table 4: Effects of IMI, GAE and their co-exposure on serum activities of liver function markers.
| Item | ALT(U/L) | AST(U/L) | TP(g/dl) | Alb(g/dl) | Glu(g/dl) |
| Control |
27.26±0.12c |
64.30±0.85bc |
6.9±0.22a |
4.08±0.17a |
2.82±0.07a |
| GAE |
27.13±0.03c |
61.63±1.10c |
6.8±0.23a |
4.1± 0.12a |
2.7± 0.21a |
| IMI |
47.36±0.23a |
86.63±1.72a |
3.48±0.08d |
1.88±0.10d |
1.59±0.23d |
| GAE/IMI |
29.03±0.37b |
67.86±0.96b |
4.39±0.11c |
2.36±0.11c |
2.02±0.19c |
| IMI/GAE |
29.30±0.25b |
66.86±1.85bc |
4.60±0.17c |
2.21±0.13c |
2.39±0.11b |
| IMI+GAE |
27.23±0.08c |
63.53±1.29bc |
5.5±0.21b |
2.9±0.09b |
2.25±0.08b |
| Sig. | ** | ** | ** | ** | ** |
Means within each column carrying different superscript are significant at (P ≤ 0.01); Star value: ** means significant at (P ≤ 0.01); ALT: alanine amino transferase, AST: aspartate amino transferase, TP: total proteins, Alb: albumin, Glu: globulin.
Table 5: Lesions score among different experimental groups.
| Organ | Lesions | Control | GAE | IMI | GAE/IMI | IMI/GAE | IMI+GAE |
| liver | Hepatitis (inflammatory cells infiltrations) | - | - | +++ | + | + | - |
| Congested blood vessels | - | - | +++ | + | ++ | + |
feeding behavior of control rats. There were no significant changes in mortality percentage among all treated groups.
Effects of IMI, GAE and their co-administration on body weight change
IMI administration significantly decreases the body weight gain of exposed rats (22.01±6.65) (p <0.05) during the entire experimental period compared with control and other treated groups (Table 3) However, simultaneous administration of GAE with IMI (combination group) or GAE alone could maintain the body gain of rats similar to control value. Moreover, the protective (GAE/IMI) and treatment (IMI/GAE) uses of GAE could enhance the gain of animals however did not reach the control values.
Effects of IMI, GAE and their co-administration on liver function parameters
ALT and AST activities were significantly elevated in rats treated with IMI than the control rats. The protection and treatment with GAE in combination with IMI (GAE/IMI, IMI/GAE, respectively) significantly decreased the activity of ALT compared to IMI alone however still higher than control values.
The protected, treated and combination uses of GAE with IMI decreased the activities of AST to the control value, however, treated and combination uses of GAE were better than the protective use in restoring AST activity to control level. Regarding TP, albumin, and globulin values, they significantly diminished in rats treated with IMI compared to control group. While their values significantly increased in GAE/IMI and IMI/GAE than IMI group with a more significant increase in IMI+GAE group however all treatments did not reach control values (Table 4).
Effects of IMI, GAE and their co-administration on the relative expression of IL6, TNF-α and TLR2 genes in liver
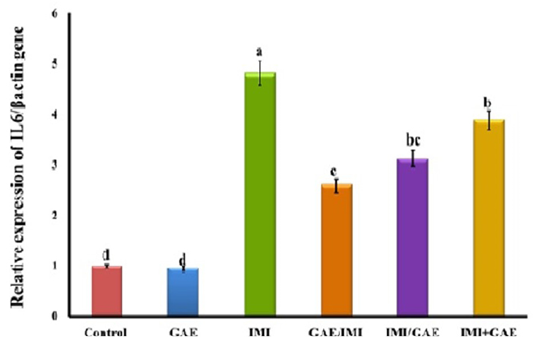
The obtained RT-qPCR data showed that there is no significant change in IL6 gene expression in the liver of control (1.00±0.06-fold change) and GAE (0.95±0.05-fold change) groups. While the expression of IL6 gene in the liver of IMI-intoxicated rats was significantly upregulated compared to control group. GAE/IMI (protected) (2.60±0.12-fold change) and IMI/GAE (treated) (3.12±0.11-fold change) significantly downregulated the expression of IL6 gene than IMI and a more pronounced downregulation in the combination group (IMI+GAE) (3.89±0.15-fold change). However, none of these treatments modulated the expression to the normal levels and remained significantly higher than that in control (Figure 1).
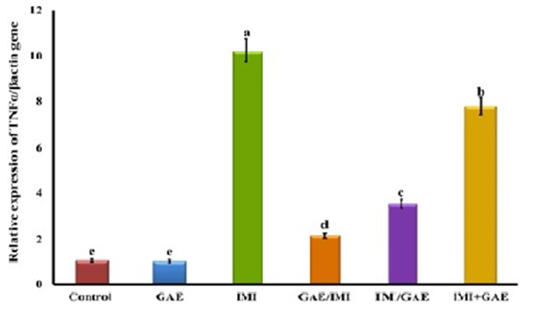
Also, the results showed that the expression of TNFα gene did not significantly change in GAE (0.97±0.06-fold change) and control group (1.00±0.08-fold change). On the other hand, IMI significantly (P≤0.05) upregulated TNFα gene expression (10.20±0.4-fold change) as compared to the control group. The protective use of GAE (GAE/ IMI) (2.16±0.12-fold change) significantly downregulated the TNFα gene expression more than the treated (IMI/GAE) (3.51±0.17-fold change) and combination (IMI+GAE) (7.78±0.32-fold change) groups compared to IMI group however the both groups did not reach to the control level (Figure 2).
The obtained data revealed significant (P≤0.05) upregulation in TLR2 gene expression in the liver of IMI-intoxicated rats (3.58±0.19-fold change) compared to control (1.00±0.07-fold change). There is no significant change between GAE (1.30±0.08-fold change) and control group. The increased expression of TLR2 obtained by IMI was significantly and similarly downregulated in the protected (GAE/IMI) (2.55±0.11-fold change), treated (IMI/GAE) (2.83±0.1-fold change) and combination group (IMI+GAE) (2.87±0.13-fold change). However, none of these treatments returned the expression to the normal levels and remained significantly higher than that in control (Figure 3).
Immunohistochemical investigations
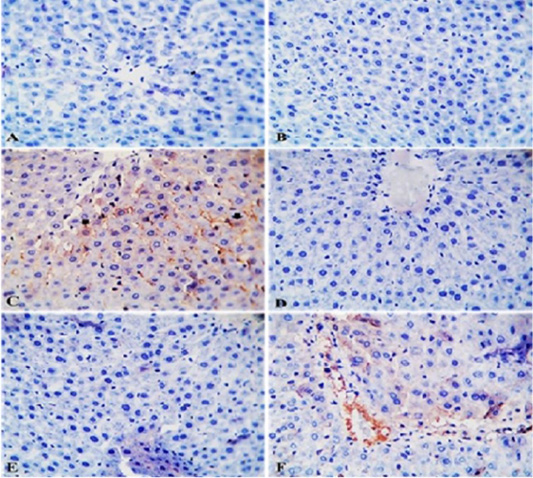
Effects of IMI, GAE and their co-administration on immunostaining reaction of TNFα in the liver tissue of rats: TNFα- IHC stained sections of liver from control rats and rats from GAE group showing normal hepatic parenchyma with negative immunostaining (Figure 4A and 4B). TNFα- IHC stained sections of liver from IMI group showing mild to moderate immunopositive staining as brownish granular materials in hepatocytoplasm (Figure 4C). TNFα- IHC stained sections of liver from GAE/IMI (protected) and IMI/GAE (treated) groups showing negative immunostaining (Figure 4D and 4E). TNFα- IHC stained sections of liver from IMI+GAE (combination) group showing mild immunostaining in a few hepatic cytoplasms.
Effects of IMI, GAE and their co-administration on immunostaining reaction of TLR2 in liver of rats: TLR2- IHC stained sections of liver from control rats and rats from GAE group showing normal hepatic parenchyma with negative immunostaining (Figure 5A and Figure 5B). TLR2- IHC stained sections of liver from IMI group showing intense immunopositive staining as brownish granular materials in hepatocytoplasm (Figure 5C). TLR2- IHC stained sections of liver from GAE/IMI (protected) group showing mild to moderate immunopositive staining in individual hepatocytes (Figure 5D). TLR2- IHC stained sections of liver from IMI/GAE (treated) group showing moderate immunopositive staining (Figure 5E). TLR2- IHC stained sections of liver from IMI+GAE (combination) showing slight immunopositive cytoplasmic granules staining in a few hepatocytes (Figure 5F).
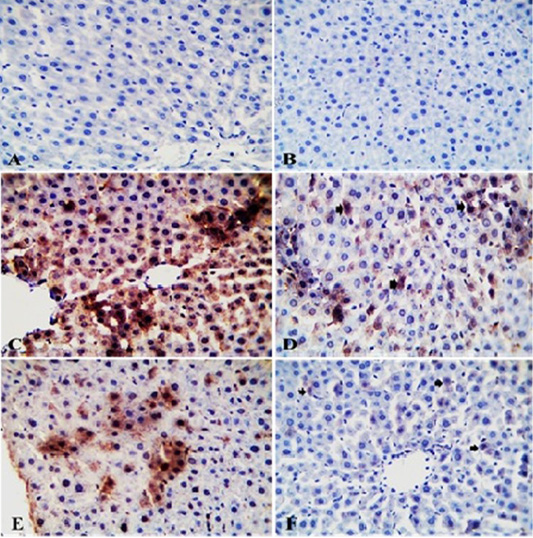
Figure 5: Photomicrograph of TLR2-IHC stained liver sections from control (A) and GAE groups (B) showing normal hepatic parenchyma with negative immunostaining. Liver sections from IMI group showing intense immunopositive staining as brownish granular materials in hepatocytoplasm (C). Liver sections from GAE/IMI (D) and IMI/GAE (E) groups showing mild to moderate immunopositive staining in individual hepatocytes (arrows). Liver sections from IMI+GAE group showing slight immunopositive cytoplasmic granules staining in a few hepatocytes (arrows) (F). X400. H and E.
Histopathological investigations
Liver of control rats and GAE rats appeared normal with preserved histomorphological structures (Figure 6A and Figure 6B). Liver of IMI treated rats revealed focal interstitial round cell aggregations which represented by disorganization of hepatic cords (replacement of hepatic parenchyma) which surrounded by lymphocytes, mild congestion of blood vessels (Figure 6C). While, liver of rats from GAE/ IMI (protected) group showed absolutely normal hepatic parenchyma with very mild round cells infiltrations in the portal areas (Figure 6D). Liver of IMI/GAE (treated) group showing most of the hepatic parenchyma, portal and stromal structures were normal whereas, mild congestion of the portal blood vessels (Figure 6E). Liver of rats from IMI+GAE (combination) group showing most of the examined sections of the hepatic parenchyma, portal trades and stromal elements were within the normal structure. Mild congested portal artery with widening vein and residual biliary and fibrotic changes were seen (Figure 6F).
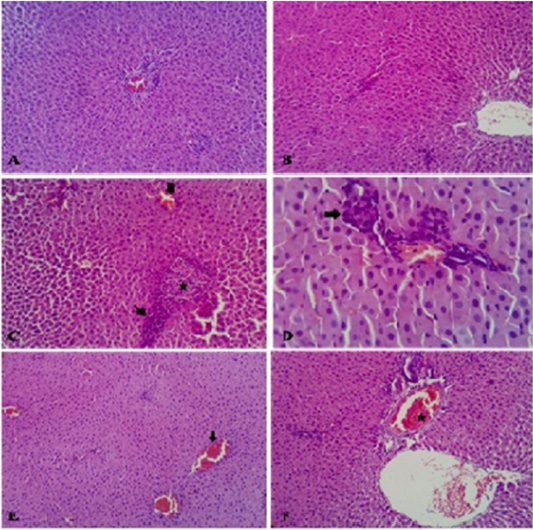
Figure 6: Photomicrograph of HandE stained liver from control rats showed normal hepatic parenchyma (A). Liver of rats from GAE group showed normal hepatic parenchyma (B). Liver of rats from IMI showed disorganization of hepatic cords replaced by mononuclear cells (star) which surrounded by lymphocytes (thin arrow) beside mild congested blood vessels (thick arrow) (C). Liver of rats from GAE/IMI showed mild portal inflammatory cells aggregations (D). Liver of rats from IMI/GAE showed normal hepatic parenchyma with mild congestion of the blood vessels (arrow) (E). Liver of rats from IMI+GAE group showed mild congested portal artery (star) with widening vein (F). H and E stain, (X 200).
DISCUSSION
IMI exposure caused significant increase in serum ALT, AST levels, significant decrease in total proteins, albumin and globulins, IMI upregulates IL6, TNF-α and TLR2 genes in the liver of rats, intense immuno positive reactivity of TLR2 and TNF α in the liver. Significant alterations in the liver tissue such as disorganization of hepatic cords replaced by mononuclear cells which surrounded by lymphocytes. Mild congestion of blood vessels was also seen. GAE administration could modulate the hepatotoxic effect of IMI.
Liver plays a major function in metabolism and performs pivotal roles as detoxification, so to assess liver injury serum enzymes like Alanine aminotransaminase (ALT), Aspartate aminotransferase (AST) were monitored (Duzguner and Erdogan, 2012).
ALT is principally important in evaluating hepatic necrosis, specifically in small animals (Cornelius, 1989). It was deliberated as an indicator of hepatocellular destruction and generally ALT was considered a more critical marker of hepatocyte injury than AST (Oser, 1976). The increased level of ALT and AST in blood denotes cell injury and increase in the membrane permeability (Ramazzotto and Carlin, 1978) and their changed metabolism (Dinman et al., 1963).
Regarding activities of ALT and AST, there was a significant increase in their levels in rats treated with IMI than the control rats. Our results agree with Bhardwaj et al. (2010); Mohany et al., 2011; Toor et al. (2013) and disagree with Duzguner and Erdogan (2012) who suggested that chronic exposure to IMI hindered AST action whereas ALT action was unaffected. Significant elevation in plasma AST level suggested high respiratory burst and mitochondrial involvement because AST is mainly a mitochondrial enzyme. Mitochondrion makes a central function in preserving hepatocyte function and integrity, which might be hindered because of extreme physiological stress (Hassanein, 2004).
Degenerative pathologic alterations noticed in our histopathological findings in liver cells increase cell membrane permeability resulting in the release of transaminases in blood stream may be responsible for the increase of AST (Shah and Gupta, 1997). It could be concluded that increased activity of aminotransferases in rats treated with IMI may be due to liver injury, as a main site of biotransformation.
The medicinal significance of ginger can be due to the great number of bioactive elements like gingerols and shogaols, considered the main bioactive compounds of ginger (Semwal et al., 2015). The protective and treatment administration of GAE with IMI significantly decrease the activity of ALT, AST compared with IMI alone however still higher than control values. On the other hand, simultaneous use of GAE with IMI could normalize the increased ALT, AST to be comparable with control. These results agree with (Abdel-Azeem et al., 2013) who suggested pretreatment with ginger significantly reduced plasma levels of ALT, AST in acetaminophen treated rats. Co-administration of ginger with piroxicam decreased ALT, AST levels (Badawi, 2018). The antioxidant action of Z. officinale can preserve the membrane integrity and hinder the release of hepatic enzymes to serum (Ajith et al., 2007).
Regarding the TP, albumin, and globulin values, they significantly diminished in rats treated with IMI. These results are in contrary to results of (Bhardwaj et al., 2010) who reported non-significant change in their levels indicating no effect on protein metabolism (Balani et al., 2011).
Our results are in agreement with Lopez-Antia et al. (2013) who attributed such decrease in TP, albumin, globulin levels to increased protein catabolism. Decreased levels of globulin indicating that the immune competency of the animals will be easily compromised by IMI (Mondal et al., 2015). While their values significantly increased in GAE/IMI (protected) and IMI/GAE (treated) groups than IMI group with a more significant increase in IMI+GAE (combination) group however all treatments did not reach control values. Al-Amin et al. (2006) demonstrated that ginger treatment decreased protein catabolism.
The secretion of pro-inflammatory cytokines is a crucial physiologic process to regulate metabolic and immune responses during tissue regeneration, development, healing, infection or trauma, and to protect our bodies against ischemia, hemorrhage, sepsis and cancer. A precise release of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukins prompt favorable inflammatory reactions that stimulate local coagulation to restrict tissue injury (De Jonge and Ulloa, 2007).
Expression of IL6, TNF-α and TLR2 genes in liver of IMI-intoxicated rats was significantly upregulated compared to the control group which is in agreement with previous findings (Duzguner and Erdogan, 2010).
Han (2007) evidently demonstrate that endosulfan, neurotoxic organochlorine pesticide, increases the secretion of pro-inflammatory chemokines, such as IL-1b, IL-6, TNF- α and their gene expression in macrophages. Studies report that nicotine can multiply macrophages secreting IL-1b and TNF- α (Wang et al., 2004).
GAE/IMI (protective) and IMI/GAE (treated) groups showed significantly downregulation of the expression of IL6 gene than IMI and a more pronounced downregulation in the combination group (IMI+GAE).
The protective use of GAE (GAE/ IMI) significantly downregulated the TNFα gene expression more than the treatment (IMI/GAE) and combination (IMI+GAE) groups compared to IMI group however both groups did not reach to the control level.
The increased expression of TLR2 obtained by IMI was significantly and similarly downregulated in ginger-treated groups. Zingiber officinale treatment was found to inhibit the elevated cytokines’ gene expression, as IL-6 and TNF- α in the liver through suppression of hepatic nuclear factor-kappa B (NF- κB) (Saedisomeolia et al., 2019). Ginger is capable of decrease expression levels of TLR2 (Mohammad et al., 2018) and suppresses the expression of inflammatory genes in a NF-κB dependent manner (Ahn et al., 2009). NF-κB is a chief transcription factor downregulates TLR2 and 4 and contributes in the TLRs expression (Lee et al., 2012; Radman et al., 2015).
Regarding immunohistochemical activity of TLR2, liver sections of rats administered IMI for 3 months showed an intense immunopositive reaction while simultaneous use of GAE with IMI (combination group) showed slight immunopositive reaction in comparison to other ginger-treated group. TNFα- immunohistochemically stained sections of liver from IMI group showed moderate immunopositive staining while Liver from GAE/IMI (protective) and IMI/GAE (treated) groups showed negative immunostaining. Co-administration of IMI and GAE (combination group) showing mild immunostaining in a few hepatic cytoplasms. The intense immunopositive reactivity of TLR2 and TNFα in the liver revealed in our results could be explained by the upregulation TLR2 and TNFα in the liver in this study.
Histopathological findings in the liver of IMI-treated albino rats for 90 days, the liver revealed focal interstitial round cell aggregations which represented by disorganization of hepatic cords (replacement of hepatic parenchyma) which surrounded by lymphocytes.These results agree with histological changes detected in livers of male rats (Mohany et al., 2011; Soujanya et al., 2013) and in layer chickens exposed to 139 mg/ kg of IMI (Kammon et al., 2010).
Liver of rats from all GAE-treated groups (protected, treated and combination) showed normal hepatic parenchyma with very mild round cells infiltrations in the portal areas indicating that ginger could restore the hepatotoxic effect caused by IMI. These results agree with previous studies Abdel-Azeem et al. (2013). Also co-administration of ginger with piroxicam revealed somewhat normal histological architecture of liver (Badawi, 2018).
CONCLUSION
The present study concludes that oral treatemtn of rats with IMI causing hepatotoxicity and changes in the liver architecture. However, GAE has modulated the hepatotoxic effect especially when administered simultaneously with IMI.
ACKNOWLEDGEMENTS
This work was supported by Faculty of Veterinary Medicine, Zagazig University, Egypt.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHORS CONTRIBUTION
All authors contributed in study design, sample collection, laboratory analysis, statastical analysis, manuscript writing and manuscript revision.
REFERENCES