Advances in Animal and Veterinary Sciences
Research Article
Virological and Molecular Studies on Peste Des Petits Ruminants Virus (PPRV) in Small Ruminants and Camel in Egypt between 2017 and 2018
Maged. R. Nafea1*, Mohamed Elbakry2, Momtaz Shahein1, Gamelat K. Farag2, Fatma Abdallah2, Ahmed A.H. Ali2
1Animal Health Research Institute, Dokki, Egypt; 2Department of Virology, Faculty of Veterinary medicine, Zagazig University, Egypt.
Abstract | Peste des petitis ruminant (PPR) is a transboundary viral disease affects mainly goats and sheep with high morbidity and high mortality rates. Thirty-Three samples were collected from small ruminant animals located within three Egyptian governorate, Sharkia, Kafr El-sheikh and Marsa-Matrouh between 2017 and 2018. Likewise, camels were included to investigate the prevalence of PPRV infection in Egypt, 103 samples were collected from three different governorates (Giza, Sharkiya and Red sea). PPRV antigens were examined in small ruminant samples using C-ELISA. For further confirmation, tissue sample from intestine of small ruminant animals subjected to real time reverse transcriptase polymerase chain reaction (RT-qPCR) and positive tissue sample were used for isolation of PPRV on Vero cells, which revealed remarkable cytopathic effect that increased obviously after three successive passages. Sera samples were subjected for C-ELISA to determine the presence of antibodies against PPRV which revealed that eight samples (8/10; 80%) were positive. Antibodies were detected in all goat three samples (100%), while in 5 sheep sera samples (5/7; 71.4%) were positive. All camel sera were negative using C-ELISA. Our results revealed that PPRV is still circulating in Egypt which leads to outbreaks in its major host (small ruminants) without spillover to camel. Therefore, effective PPR vaccination program is recommended to be applied regularly in Egypt with strict quarantine measures at the borders to prevent the introduction of new PPRV genotypes to the Egyptian industry.
Keywords | Peste des petits ruminants virus, C-ELISA, RT-qPCR, Sheep, Goat, Egypt
Received | September 11, 2019; Accepted | October 06, 2019; Published | October 10, 2019
*Correspondence | Maged. R. Nafea, Animal Health Research Institute, Dokki, Egypt; Email: [email protected]
Citation | Nafea MR, Elbakry M, Shahein M, Farag GK, Abdallah F, Ali AAH (2019). Virological and molecular studies on peste des petits ruminants virus (PPRV) in small ruminants and camel in Egypt between 2017 and 2018. Adv. Anim. Vet. Sci. 7(s2): 12-18.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s2.12.18
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Nafea et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Peste des petitis ruminants (PPR) is an acute highly contagious pneumo-enteric transboundary viral disease specific affecting small ruminants and small wild stocks and manifested by fever, respiratory signs and intestinal signs (Cam et al., 2005). The causative agent is PPRV, which has been classified in order Mononegavirales, family Paramyxoviridae, subfamily Orthoparamyxovirinae, genus Morbillivirus (ICTV, 2018). PPRV has an enveloped, spherical and intact virion with pleomorphic negative sense single stranded RNA (Barrett et al., 2005; Woo et al., 2012). PPR viral genome has 6 genes (3’ N-P-M-F-H-L 5’), which encodes for six structural PPRV proteins (Munir et al., 2012). PPRV was first reported in Côte d’Ivoire, subsequently in most African countries, Middle East countries, Turkey; Arabian Peninsula then spreads widely from Central Asia to South and South East Asia (Banyard et al., 2010). Europe maintain PPRV disease surveillance due to reporting of outbreaks on the Edges of the European Union with Morocco and Tunisia (Minet et al., 2009). PPR has a major economic effect due to having an impact on the food security and economic livestock trade of the affected country especially in developing countries (Wambura, 2000; Banyard et al., 2010). In Egypt, PPRV has been reported since 1989 with very low incidence then later PPR has been one of the threatening diseases (Ismail et al., 1990; El-Hakim, 2006; Abd El-Rahim et al., 2010; Ayman, 2017; Hend et al., 2019).
PPRV has only one serotype but based on the sequence analysis of its nucleoprotein (N) gene there are at least 4 lineages (Geerts, 2009). PPRV is genetically related to measles (Me), rinderpest (RP), and canine distemper (CD) viruses. Antibodies against PPRV can cross-neutralizing and cross protective against RPV (Taylor, 1979). The most similar genome with PPRV is RPV on the level of nucleotide sequences similarities (Bailey et al., 2005). PPRV lineage I and II has been identified in Central Africa and West Africa; Ivory Coast, Guinea and Burkina Faso (Luka et al., 2011; Munir et al., 2012). Lineage III isolated from East Africa, Sudan, Yemen and Oman (Mahapatra et al., 2015). Recently, Lineage IV isolated from Arabian Peninsula, Middle East, Southern Asia, and across several African territories (De Nardi et al., 2012; Libeau et al., 2014). In spite of, sheep and goats display the clinical form of PPR disease; PPRV has been reported in other wild species especially in captive wild ungulates (Abu Elzein et al., 2004; Kinne et al., 2010). Goats were reported to display more severe symptoms and outbreaks than sheep (Khan et al., 2007; Abubakar et al., 2008). Some studies reported that subclinical form of the disease might occur in other large ruminants (cattle and buffalo) and pigs, which play an important role in dissemination and circulation PPRV (Hamdy and Daridi, 1976; OIE, 2012). Previous serological surveys revealed that the susceptibility of camels to PPR infection with probability of expressing a serious illness represented by respiratory distress and death (Haroun et al., 2002) which are the same as those observed in small ruminants with slight variations (Khalafalla et al., 2010; Kwiatek et al., 2011; Zakian et al., 2016; Omani et al., 2019). PPRV is transmitted through close contact between infected and susceptible animals through shedding after a relatively short period following infection. The main route of transmission is aerosols through nuzzling and licking between infected and susceptible animals as well as oral route or contact with secretions and excretions of infected animals (Geerts, 2009).
The present study aims to detect PPRV based on serological and molecular investigations in small ruminant animals and camels in some Egyptian governorates.
MATERIAL AND METHODS
Sampling history
PPRV suspected outbreaks were recognized in sheep and goats farms with no any vaccination history in distinctive Egyptian governorates including Sharkia, Kafr El-Sheikh and Marsa Matrouh have been reported during 2017-2018 through manifestation of the standard signs of the disease including; anorexia, pyrexia, profuse serous oculonasal discharges and diarrhea. High morbidity and mortality rates were reported in different ages. The outbreaks were involved mainly goats and to lesser extent sheep. A total number of 33 samples from suspected PPR outbreaks were collected (16 sheep and 17 goats). The samples include 16 swabs (9 nasal, 2 rectal,4 oral,1occular) and 5 buffy coat samples collected from animals displaying the clinical signs of PPRV infection with severe symptoms in both goats than sheep. Two tissue samples (lung tissue and intestinal mucosa tissue) were collected from necropsied goat and 10 serum samples from apparently healthy animals in contact with diseased animals. Additionally, 103 samples were collected from camels from three different governorates (Giza, Skarkia and Red sea) including; 10 nasal swabs and 83 serum samples which were collected from apparently healthy live camels while 10 pneumonic lung tissue samples were collected post slaughtering at abattoirs (Table 1).
Samples processing and preparation
In the laboratory, tubes containing whole blood with EDTA as anticoagulant were centrifuged at 2000 rpm / 10 minutes. Plasma was collected in Eppendorf and then sterile distilled water was added to the samples for 50 sec to hemolyse RBCs then double volume of PBS was added. The tubes were centrifuged at 2000 rpm /10 minutes. Washing with PBS was applied for 3 to 4 times to obtain clear buffy coat. Sera samples were collected from diseased and apparently healthy animals and collected in Eppendorf and freezed at -80°C till used. Nasal swabs were collected in tubes containing PBS with antibiotic mixture as a transport media, swabs were vortexed for 60 seconds, centrifuged at 3000 rpm/ 10 minutes then the sediment was discarded and the supernatant was collected in eppendorf and freezed at -80oC till used. Tissue samples (Intestine and lung tissue) of necropsied PPR suspected small ruminant animals were collected freshly after death and used later for PPRV antigen detection and PPRV isolation. While, pneumonic lung samples were collected aseptically from camels after slaughter and used later for PPRV antigen detection, identification and isolation (Table 1).
PPRV antigen detection using an immunocapture ELISA (IC-ELISA) assay
Immunocapture ELISA assay using IC-ELISA Kit (CIRAD EMVT, Montpellier, France) was used for detection of PPRV antigen in the tested samples following the manufacturer’s instructions.
PPRV antibodies detection using a competitive ELISA (C-ELISA) assay
Competitive screening ELISA Kit (ID Vet Innovative Diagnostics, France) was performed for detection of PPRV antibodies in the suspected sera using monoclonal
Table 1: Sampling data from Small ruminants and Camel in some governorates of Egypt during years 2017-2018.
| Type of sample | Species | Number of collected samples | Governorate | Date of collection | Total number of samples | |
| Small ruminants | Small ruminants | Camel | ||||
| Buffy coat | 3 | Zagazig Sharkiya |
February 2017 |
5 | ||
| 2 | Kafr El Sheikh | February 2017 | ||||
| Sera | Camel | 22 | Shalateen– Red Sea | March 2018 | 10 | 83 |
| 33 | Shalateen– Red Sea. | May 2018 | ||||
| 14 | Shalateen– Red Sea. | July 2018 | ||||
| 14 | Burqash-Giza | July 2018 | ||||
| Small ruminants | 3 | Kafr El Sheikh | February 2017 | |||
| 9 | Marsa Matrouh | April 2017 | ||||
| Rectal swabs | Small ruminants | 2 | Kafr El Sheikh | March 2017 | 2 | |
| Nasal swabs | Camel | 5 | El-Marg Abattoir – Cairo | Januanry 2018 | 10 | |
| 5 | Elaslogy Abattoir – Sharkia | April 2018 | ||||
| Small ruminants | 2 | Sharkia | March 2017 | 9 | ||
| 3 | Kafr El Sheikh | February 2017 | ||||
| 4 | Marsa Matrouh | April 2017 | ||||
| Oral swabs | Small ruminants | 2 | Sharkia | February 2017 | 4 | |
| 2 | Kafr El Sheikh | February 2017 | ||||
| Ocular swabs | Small ruminants | 1 | Kafr El Sheikh | February 2017 | 1 | |
| Tissue samples | Camel | 5 lung tissue | El-Marg Abattoir – Cairo | March 2018 | 10 | |
| 5 lung tissue | Elaslogy Abattoir – Sharkia | April 2018 | ||||
| Small ruminants | 1 intestine 1 Lung | Marsa Matrouh | April 2017 | 2 | ||
| Total number | 136 | 33 | 103 | |||
antibodies directed against PPRV nucleoprotein gene following the manufacturer’s instructions.
Isolation of PPRV on African green monkey kidney cell culture (Vero cells)
Tissue samples were used for isolation of PPRV on Vero cells (ATCC-CCL-81) (supplied by Animal Health Research Institute, Dokki, Giza, Egypt) by three successive blind passages for PPRV which resulted in remarkable cytopathic effect (Ahmed, 2006; Kumara et al., 2013).
Detection of PPRV using real time quantitative reverse transcription polymerase chain reaction (RT-qPCR)
An immunocapture antigen ELISA positive sample (tissue sample from intestine of necropized goat), PPRV infected Vero cell culture inoculated with the same sample and all camel antigens negative ELISA samples were examined with RT-qPCR for confirmation of negativity by detection of PPRV RNA. RNA extraction was done using RNA extraction kit (Thermo Scientific Gene JET RNA Purification kit) as per the manufacturer’s instructions. RT-qPCR according to Primerdesign TM Ltd Peste-des-petits- ruminants virus large protein gene genesig® Advanced Kit handbook (Couacy-Hymann et al., 2002).
RESULTS
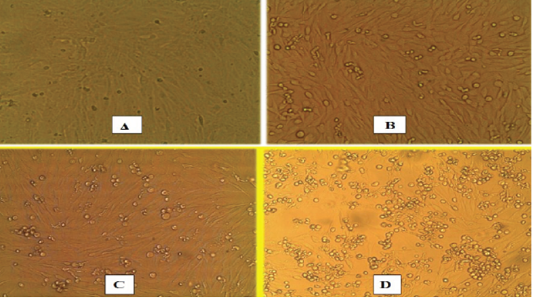
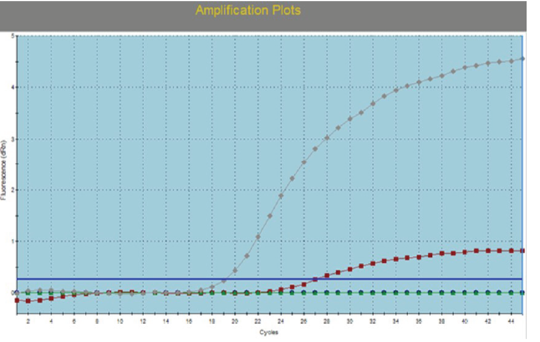
Swabs (Nasal, ocular, oral, rectal), buffy coat, tissue samples collected from small ruminant animals were tested with IC-ELISA for identification of PPRV antigen which was detected in 16/23, 69.5% from the tested samples, while all samples (nasal swabs and lung tissues) of camels were negative (Table 2). For further confirmation of IC-ELISA positive samples, tissue sample from intestine of necropsied animals subjected to RT-qPCR for RNA amplification. While all negative antigen tested camel samples were confirmed to be negative using RT-qPCR. For further PPRV identification, the positive tissue samples were used for isolation of PPRV on Vero cells. Three successive blind passages for the virus on Vero cells till CPE became noticed starting from 3rd, 4th and 5th day post inoculation. CPE increased obviously till the 3rd passage with characteristic cytopathic effect (CPE); rounding, shrinkage, detachment, gapping and cluster aggregations of Vero cells (Figure 1). The PPRV Vero infected cells subjected later for RT-qPCR for detection of PPRV whereas the mean Ct value of the reference genes ranged from 18 to 24 (Figure 2). For detection of anti-PPRV antibodies, sera samples were subjected to C-ELISA, which revealed positive antibodies in 8/10, 80% out of the examined sera samples of sheep and goats (Table 3).
Table 2: PPRV antigen detection and identification using an immunocapture ELISA (IC-ELISA) assay.
| C-ELISAresults | No. of samples | Species | Type of samples | |
|
2/6)) Ve+ |
6 | sheep |
small ruminants |
Nasal swab |
|
Ve(3/3) + |
3 | goat | ||
| -Ve | 10 | camel | ||
| - | - | goat |
small ruminants |
Tissue samples |
|
Ve(1/2)+ |
2 | |||
| -Ve | 10 | camel | ||
|
(1/1) +Ve |
1 | sheep |
small ruminants |
Oral swab |
|
Ve(3/3)+ |
3 | goat | ||
| +Ve(2/2) | 2 | goat |
small ruminants |
Rectal swab |
| +Ve(1/1) | 1 | Ocular swab | ||
| +Ve(3/5) | 5 | Buffy coat | ||
Table 3: PPRV antibodies detection and identification using a competitive ELISA (C-ELISA) assay.
| C-ELISAresults | No. of samples | Species | Type of samples | |
|
Ve(5/7)+ |
7 | Sheep |
Small ruminants |
Serum |
|
Ve(3/3)+ |
3 | goat | ||
| -Ve | 83 | Camel | ||
DISCUSSION
In developing countries, especially in the humid tropics, small ruminants production plays an important economic impact (Boyazoglu et al., 2005). PPRV has been considereda major threat to small ruminant production and affects the livelihood of farmers (Cam et al., 2005; Dialloet al., 2007; Banyard et al., 2010). Camels have been demonstrated to be susceptible to the infection and may express serious manifestation in some cases as respiratory distress, paralysis have been also observed and death (Haroun et al., 2002; Khalafalla et al., 2010; Zakian et al., 2016).
Camels also can be a source of PPRV infection for ruminants especially sheep and goats due to nature of husbandry system allowing camels to get contact with other ruminants at points of grazing, watering and market places (Libeau et al., 1995). Egypt is exposed to high risk of PPR spread due to the history of PPRV in Africa, existing small ruminant trading, uncontrolled animal movement and bordering nations as well as the transboundary nature of PPR.
For detection of anti-PPRV antibodies, sera samples were used for C-ELISA which revealed antibodies detection in 8/10 (80%) tested sera samples of the examined sheep and goats. All goat sera have higher antibodies seroprevalence (3/3, 100%) in compared with sheep sera (5/7, 71.4%) which is in concordance with a previous findings which reported the higher prevalence of PPRV in goat than sheep (Saed et al., 2017). In our study, only one antigen positive tissue sample from intestine of necropized goat is examined with RT-qPCR which confirmed to be positive. For further identification, PPRV was isolated from field tissue samples on Vero cells which succeed after three successive blind passages, with characteristic CPE (OIE, 2013). Recently, PPRV is the cause of fatal respiratory disease in camels in Sudan, in spite of sheep and goats are the main host of PPRV infection (El-Hag Ali et al., 1984; Haroun et al., 2002).
To investigate the probability of circulation and dissemination of PPRV from camels that have been reported to play a role in transmission of PPRV. Sera samples were collected from camels then tested by C-ELISA, which were negative. However, previous studies reported the detection of PPRV antibodies in camel serum (Haroun et al., 2002; Abraham et al., 2005). Also, Nasal swabs and lung tissue samples of camels were negative when examined using IC-ELISA for PPRV antigen detection. The data obtained from this study revealed that PPRV is still a leading cause of outbreaks and high fatalities with an alarming increase in disease rate including newer areas in Egypt. The reported clinical cases were never being vaccinated against PPR. Furthermore, there is an exclusion of the circulation and spillover of PPRV to camels in spite of reporting and isolation PPRV from camel in neighboring African countries. There are important measures suggested to control PPR as movement restriction of sheep and goats from affected areas, quarantine of affected animals and elimination of contact fomites as well as disinfection of the affected premises. However, vaccination is still the most effective means for controlling the disease. Therefore, effective PPR mass vaccination program is highly recommended to apply regularly in Egypt according to OIE/FAO global pathway for PPR eradication
CONCLUSION
The epidemiological situation of PPRV in Egypt is poorly understood due to little studies on PPRV in Egypt while exposing to high risk of PPR as a result of transboundary nature of the virus , uncontrolled movement of the animals and small ruminant animals trade between Egypt and neighboring countries especially Sudan. Laboratory diagnostic abilities should be improved for early detection and control of disease due to subclinical form of PPRV infection that may occur and can be only identified by the detection of PPR antibodies in apparently healthy animals during sero-surveillance. Egyptian veterinary medical authorities should apply a regular vaccination program to protect highly susceptible animals from PPRV. Effective PPR mass Vaccination program according to OIE/FAO global pathway for PPRV eradication is recommended to be used covering all the Egyptian governorates. Restriction for animal movement and quarantine should be applied to prevent the incidence of the disease spread and outbreaks. Until now, there are no accessible DIVA tests for PPRV detection, so we are in a need for DIVA diagnostic tests and marker PPR vaccines to differentiate between field wild-type and vaccine PPRV strains.
ACKNOWLEDGEMENTS
This work was supported by Animal Health Research Institute, Dokki, Egypt, and Faculty of Veterinary Medicine, Zagazig University, Egypt.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHORS CONTRIBUTION
All authors contributed equally in this work.
REFERENCES