Advances in Animal and Veterinary Sciences
Research Article
Incidence of Aerococcus viridans in Raw Cow Milk in Sohag City, Egypt
Eman M. Shaker1, Alshimaa A. Hassanien2, Esraa Y. Abd-Elhamed1
1Department of Food Hygiene, Faculty of Veterinary Medicine, Sohag University, Egypt; 2Department of Zoonoses, Faculty of Veterinary Medicine, Sohag University, Egypt.
Abstract | Aerococcus viridans considered the second bacterial cause of mastitis in bovine with unclear pathogenic changes role. Therefore, the current study was conducted to investigate incidence of subclinical mastitis and A. viridans in 100 raw milk samples collected from different dairy cattle breeding farms in Sohag city, Egypt, its effect on some milk composition and their antibiotic resistance was described. Subclinical mastitis was detected in high incidence rate (92%). A total of eleven A. viridans isolates were identified from 92 bovine subclinical mastitis cases. Comparatively with milk of healthy cows, the mean chloride content (%) of infected milk was 0.110 ± 00013, which showed highly significant (P = 0.01) increase, while, the mean lactose (%) decreased significantly. All A. viridans isolates were 100% susceptible to Streptomycin, Amikacin and Ciprofloxacin, and followed by (90.91%) to Vancomycin while, all A. viridans isolates were highly resistant to Penicillin G, Ampicillin and Cefotaxime. This study concluded that A. viridans play an important role in subclinical mastitis infection in bovine in Sohag city, where it exerts an effect on some milk composition and contaminated milk considered as a hazard for human health.
Keywords | Subclinical mastitis, Aerococcus, A. viridans, Sohag, Egypt.
Received | May 23, 2019; Accepted | July 28, 2019; Published | August 26, 2019
*Correspondence | Eman M Shaker, Department of Food Hygiene, Faculty of Veterinary Medicine, Sohag University, Egypt; Email: [email protected]
Citation | Shaker EM, Hassanien AA, Abd-Elhamed EY (2019). Incidence of aerococcus viridans in raw cow milk in sohag city, egypt. Adv. Anim. Vet. Sci. 7(9): 782-787.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.9.782.787
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Shaker et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Bovine subclinical mastitis resulted in inflammation of mammary glands causing reduction in milk quality and quantities. It is resulted from infection with microbes which either contagious or environmental pathogens (Bakr et al. 2019). Milking process considered the main cause of infection as this microorganism living in the environment (Aguirre and Collins, 1993).
Aerococci showing identity in biochemical and physiological characters as pediococci, enterococci, lactococci and leuconostocs, and are frequently mistaken with streptococci (Facklam et al. 1989).This genus exhibit a weakly reaction with catalase test but do not contain cytochrome. Aerococcus genus primarily reported as one species named A. viridans (Williams et al., 1953). New five species of Aerococcus were additionally recognized: A. urinae, A. christensenii, A. sanguinicola, A. urinaeequi, and A. urinaehominis (Euzeby, 1997).
However, nowadays these organisms have a great importance in human and veterinary medicine (Spakova et al., 2012). A. viridans responsible for several human hazards as endocarditis, meningitis and arthritis (Gopalachar et al., 2004; Popescu et al., 2005). A. viridans was recently involved in bovine mastitis as it has been isolated from clinical and subclinical cases Spakova et al. (2012); Liu et al. (2015); Saishu et al. (2015); Sun et al. (2017), and described as the causative agent of arthritis, pneumonia and meningitis in cows (Liu et al. 2015). Among the infectious diseases of large ruminants, A. viridans still remains one of the threats to rural economy of many countries including Egypt. Few scientific literatures are available regarding the incidences in Egypt. The present study aimed to monitor the role of A. viridans in cases of subclinical mastitis in Sohag city, Egypt, its effect on some milk composition and describe their antibiotic resistance.
MATERIAL AND METHODS
Sample Collection
The study was conducted between January and August 2018 on one hundred Holstein dairy cows, apparently healthy and not received any specific treatment before study, from different dairy breeding farms in Sohag city.
Detection of Subclinical Mastitis
Examination of collected milk samples (quarter samples) for diagnosis of subclinical mastitis by strip cup test and California Mastitis Test (CMT) (Bovivet®, Kruuse™, Denmark), with subsequent collecting of individual milk samples (mixed quarters’ samples) for bacteriological examination.
Isolation of Aerococcus Species
All normal milk samples and which show subclinical mastitis by Strip cup test & CMT were subjected to isolation and identification of Aerococcus species (Sun et al., 2017).
Enrichment
One ml of each homogenized sample was aseptically inoculated into a sterile test tubes containing 10 ml of tryptone soya broth (TSB) (M011, HiMedia). The inoculated tubes were incubated at 37oC for 24 hr.
Selective Plating
A loopful of incubated broth cultures were streaked on trypticase soya agar (TSA) (M290-500G, HiMedia) with 5% sheep blood, then incubated aerobically at 37oC for 24 h. Translucent colonies with green alpha haemolytic activity were chosen for further identification according to (Liu et al. 2015).
Identification of Aerococcus species
Morphological characters: Films were made from pure cultures and stained with Gram’s stain and examined microscopically. The organism appears round Gram-positive cocci 1-2μ in diameter usually staining deeply, arranged in singles, pairs, tetrad and irregular clusters.
Biochemical reactions: All cultures that gave negative catalase reaction considered as suspected Aerococcus isolates and retained for identification by API 20 strep system (bioMérieux, SA,Marcyl’Etoile, France), the identification is performed using the database (V 7.0) with the apiwebTM identification software.
Detection of Aerococcus viridans by using PCR: Extraction of DNA was performed using the QIAamp DNA Mini kit (Qiagen, Germany, GmbH) following the manufacturer’s recommendations. Primers used were purchased from Germany (Table 1), 25- µl reaction containing 12.5 µl of Emerald Amp Max PCR Master Mix (Takara, Japan), 1 µl of each primer with, 4.5 µl water, and 6 µl DNA template. Using an applied biosystem 2720 thermal cycler. PCR conditions involved denaturation for 5 min at 94oC followed by 35 cycles of annealing at 59oC for 40 sec, extension at 72oC for 45 sec and denaturation for 30 sec at 94oC. There was a final extension at 72oC for 10 min. Samples were hold at 4oC until analyzed by agarose gel electrophoresis.
Table 1: Sequence of oligonucleotide.
| Primer | Target gene | Sequence of oligonucleotide |
Segmented (bp) |
Reference |
| AC2 | 16S rRNA | (5`- GTG CTT GCA CTT CTG ACG TTA GC-3`) | 450 bp | Martín et al., 2007 |
| AC4 | (5`-TGA GCC GTG GGC TTT CAC AT-3`) |
PCR were separated by electrophoresis using 100 bp ladder (Fermentas, Thermofisher) and photographed by a gel documentation system (Alpha Innotech, Biometra).
Effect of Aerococcus viridans on milk composition of chloride and lactose: Milk composition of chloride (%) and lactose (%) of A. viridans positive samples were determined by using automatic milk analyzer (Lactoscan MCC, Lactoscan milktronic) (Draaiyer et al., 2009) in Dairy science Department in Faculty of Agriculture, Sohag University.
Table 2: Incidence of subclinical mastitis in the examined raw milk samples
| Source of samples | No. of samples |
Normal samples |
Subclinical mastitis samples |
||
| No. | % | No. | % | ||
| Dairy farm (A) | 75 | 4 | 5.33 | 71 | 94.67 |
| Dairy farm (B) | 25 | 4 | 16 | 21 | 84 |
| Total | 100 | 8 | 8 | 92 | 92 |
Antimicrobial Susceptibility Testing
Antibiotic sensitivity of A. viridans isolates against 11 antimicrobial agents was performed utilizing the Kirby-Bauer disk diffusion method following rules of (CLSI, 2013) by applying the antibiotic sensitivity discs containing Penicillin G (10µ), Ampicillin (10µ), Amoxicillin/ Clavulanic acid (AMC) (20/10µ), Cefataxime (30µ), Tetracycline (30µ), Streptomycin (10µ), Amikan (30µ), Erythromycin
Table 3: Incidence of Aerococcus spp in the examined raw milk samples by using API
| Source of sample |
No. of samples |
No. of isolates | Suspected isolates |
Positive Aerococcus spp. from Subclinical mastitis samples by API |
|||
| Sub-clinical mastitis samples | Normal samples |
Sub-clinical mastitis samples |
Normal samples | % | No. | ||
| Dairy farm (A) |
75 |
50 | 4 | 29 | ــــــ | 30.67 | 23 |
| Dairy farm (B) |
25 |
11 | 4 | 11 | ــــــ | ــــــ |
ـــــ |
| Total |
100 |
61 | 8 |
40 |
ــــــ | 23 | 23 |
(15µ), Clindamycin (2µ), Vancomycin (30µ), Ciprofloxacin (5µ) on Muller-Hinton agar plates (Oxoid, Shanghai, China) and swabbed with the broth culture, and then incubated for 24 h at 37oC in aerobic atmosphere. Results were interpreted according to (CLSI, 2013).
Statistical Analyses
Milk composition of healthy cows and A. Viridans infected cows were compared and data statistically analysed using SPSS (SPSS 14 for windows, Inc., USA). Means and standards deviations were measured and data was significant when P value < 0.05.
RESULTS AND DISCUSSION
Incidence of Subclinical Mastitis
Examination by field tests for diagnosis of subclinical mastitis in collected raw milk samples indicated that 92% of the total examined raw milk samples were positive for mastitis represented as 71 samples from dairy farm (A) and 21 samples from dairy farm (B) as shown in Table (2). Lower results (19.4, 41.02 and 53%) were obtained by Abdel-Rady and Sayed (2009); El-kholy et al. (2018) and Bakr et al. (2019), respectively. The increased incidence of subclinical mastitis among dairy animals may be attributed mainly to poor hygiene practices, inadequate housing and bedding, malfunctioning milking machines, improper milking procedures and in adequate treatment methods (Philipot, 1984).
By using isolation method from both normal and subclinical mastitis raw milk samples, we obtained 8 isolates from normal raw milk samples and 61 isolates from subclinical mastitis milk samples. With respect to preliminary identification of Aerococcus spp, this organism distinct and different from most of other microorganisms, only 40 isolates were identified as suspected Aerococcus species which were isolated from subclinical mastitis samples only, and needed more identification, these suspected isolates were obtained as 29 isolates from dairy farm (A) and 11 from dairy farm (B) as shown in Table 3.
Incidence of different Aerococcus spp.
By applying API 20 strep system on suspected isolates it was cleared that the incidence of Aerococcus species from the total examined raw milk samples was 23% obtained from subclinical mastitis milk samples from dairy farm (A) only (Table 3), and distributed as A. viridans (11%), A. urinae (10%) and A. sanguinicola (2%) as show in Table (4). Higher results (15%) of A. viridans by API were obtained by Sukru et al. (2018) and lower results 1% and 2% of A. viridans were obtained by Spakova et al. (2012); McDonald et al. (2005), respectively.
Table 4: Incidence of different Aerococcus spp. in the examined raw milk samples by using API
|
Aerococcus species |
Number of isolates | |
| No./100 | % | |
| Aerococcus viridans | 11 | 11 |
| Aerococcus urinae | 10 | 10 |
| Aerococcus sanguinicola | 2 | 2 |
| Total | 23 | 23 |
Detection of Aerococcus viridans by using PCR
Previous investigations reported a minimal involvement of A. viridans in cases of mastitis and this could have been ascribed to an underestimation, resulting from misidentifications as streptococci or staphylococci. However, enhancements in identification techniques, particularly the introduction of molecular assays has promoted to confident detection
Table 5: Incidence of A. viridans in the examined raw milk samples
| Examined samples |
Positive A. viridans samples |
|||
| API | PCR | |||
| No./100 | % | No./100 | % | |
| Normal samples | ــــ | ــــ | ــــ |
ـــــ |
| Subclinical mastitis samples | 11 | 11.96 | 11 | 11.96 |
|
Total |
11 | 11.00 | 11 |
11.00 |
Table 6: Statistical analytical results of CMT in positive A. viridans samples
| Examined quarter | Score ± |
|
Score + | Score ++ | Score +++ | |||
| No./11 | % | No./11 | % | No./11 |
% |
No./11 |
% |
|
| FL | 1 |
9.091 |
4 | 36.363 |
2 |
18.182 | 4 | 36.363 |
| FR | 3 |
27.273 |
1 | 9.091 |
4 |
36.363 | 3 | 27.273 |
| HL | __ |
___ |
3 | 27.273 |
3 |
27.273 | 5 | 45.454 |
| HR | ___ | ___ | 2 | 18.182 |
6 |
54.545 | 3 | 27.273 |
|
Total/44 |
4 |
9.09 | 10 | 22.73 |
15 |
34.09 | 15 | 34.09 |
FL=front left, FR= front right, HL= hind left, HR= hind right
Table 7: Effect of A. viridans on milk composition as compared to healthy cows
| Milk contents |
Healthy cow ( n = 8) (mean ± SD) |
Subclinical mastitis cows
(n = 11)
(infected with A .viridans) (mean ± SD) |
* P value |
|
Chlorid % |
0.087 ± 0.005 | 0.110 ± 0.013 |
0.01 |
| Lactose % | 4.78 ± 0.2 | 3.06 ± 0.3 |
0.01 |
*P value is highly significant at level of 0.01
Table 8: Antibiotic susceptibility profile of A. viridans isolates using Kirby-Bauer disk diffusion method
| Antimicrobials | Concentration |
Breakpoints |
ZIDa (mm) |
S (%) |
|
I (%) |
|
R (%) |
|
||
|
S |
I | R |
No./11 |
% | No./11 | % | No./11 |
% |
|||
| Penicillin G | 10 IU | 22 ≤ | 19-21 | 18 ≤ | 9-15 | ---- | ---- | ---- | ---- | 11 | 100 |
| Ampicillin | 10 µg | 17 ≤ | --- | 16 ≤ | 7-10 | ---- | ---- | ---- | ---- | 11 | 100 |
| Amoxicillin/Clavulanic acid (AMC) | 30 µg | 20 ≤ | --- | 19 ≤ | 12-23 | 2 | 18.18 | ---- | ---- | 9 | 81.82 |
| Cefotaxime | 30 µg | 28 ≤ | 26-27 | 25 ≤ | 8-15 | ---- | ---- | ---- | ---- | 11 | 100 |
| Tetracycline | 30 µg | 23 ≤ | 19-22 | 18 ≤ | 15-20 | ---- | ---- | 4 | 36.36 | 7 | 63.63 |
| Streptomycin | 10 µg | 18 ≤ | 14-17 | 13 ≤ | 19-39 | 11 | 100 | ---- | ---- | ---- | ---- |
| Amikacin | 30 µg | 17 ≤ | 15-16 | 14 ≤ | 25-38 | 11 | 100 | ---- | ---- | ---- | ---- |
| Erythromycin | 15 µg | 21 ≤ | 16-20 | 15 ≤ | 17-28 | 5 | 45.45 | 6 | 54.54 | ---- | ---- |
| Clindamycin | 2 µg | 19 ≤ | 16-18 | 15 ≤ | 13-20 | 6 | 54.54 | 4 | 36.36 | 1 | 9.09 |
| Vancomycin | 30 µg |
17 ≤ |
---- |
---- |
16-22 |
10 |
90.91 |
1 |
9.09 |
---- |
---- |
| Ciprofloxacin |
5 µg |
16 ≤ |
13-15 |
12 ≤ |
20-32 |
11 |
100 |
---- |
---- |
---- |
---- |
S -susceptible, I- intermediate, R – resistant aZone of inhibition range
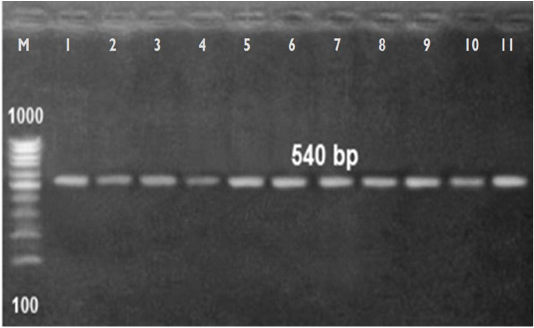
of A. viridans. The eleven A. viridans isolates detected by API examination were finally confirmed by 16S ribosomal RNA (rRNA) sequencing as A. viridans (Table 5 & Figure 1). Notably, recovery of A. viridans from milk of cows showing subclinical mastitis referred to its role as an environmental pathogen resulted in bovine subclinical mastitis. Liu et al. (2015) and Saishu et al. (2015) isolated A. viridans in pure culture from cows with subclinical mastitis in percentages of 6.1% and 8 %, respectively.
The degree of quarter attack due to A. viridans infection was varied from 15 quarters (34.09%) showed degree (+++), 15 (34.09%) showed degree (++), 10 (22.73%) showed degree (+), 4 (9.09%) showed degree (±), as shown in Table 6. From previous result we found that the highest degrees of quarter attack were more in hind quarters than in fore quarters which may be due to the morphological structure of the udder and their proximity to the rear of animal which considered as a source of contamination.
Effect of A. viridans on Milk Composition of Chloride and Lactose
Table 7 shows describe the changes caused by A. viridans on some milk constituents compared to healthy cow milk. Mean chloride percent of subclinical mastitis milk was 0.110±0.013 showing high significant (P<0.01), while, the mean lactose (%) decreased significantly. The reduction in lactose content in milk infected with A. viridans was also observed by Sun et al. (2017).
Antimicrobial Susceptibility Profile
A. viridans isolates which isolated from bovine mastitis from different geographical areas Martin et al. (2007); Špaková et al. (2012); Sukru et al. (2018) were highly diverse in their antibiotic resistance patterns. Table 8 Determine the effect of some antibiotics against 11 A. viridans isolates. All A. viridans isolates were 100% susceptible to Streptomycin, Amikacin and Ciprofloxacin, and highly susceptible (90.91%) to Vancomycin but only 5 (45.45%) and 6 (54.54%) A. viridans isolates were susceptible to Erythromycin and Clindamycin respectively. On the other hand all A. viridans isolates were highly resistant to Penicillin G, Ampicillin, Cefotaxime and majority of isolates were resistant to Amoxicillin/Clavulanic acid (AMC) (81.82%) and Tetracycline (63.63%).
The same results were reported by Špaková et al. (2012), particularly for the resistance patterns of beta lactamase resistanse while, Martin et al. (2007) showing a different results as he found that all A.viridans isolates were susceptible to B-lactamase antibiotics. The resistant of A. viridans for some commercial antibiotics which used commonly in different programs of animal and human treatment lower the efficacy of antibiotics against infections and attributed to the hazards for human when transmitted through milk consumption.
CONCLUSIONS
From our results A. viridans play an important role as a causative agent of subclinical mastitis in cows and milk considered as a hazard for human infections with A. viridans through milk consumption. Hence understanding of epidemiology and risk factors is highly essential in order to formulate appropriate management programs.
CONFLICT OF INTERESTS
None.
AUTHORS CONTRIBUTION
Contribution is equal to all authors
REFERENCES