Advances in Animal and Veterinary Sciences
Review Article
Molecular and Serological Characterization of Toxoplasma gondii in Women in Wasit Province
Abbas Hassan Al-Sray1, Sarhan Rashid Sarhan2*, Hussein Ali Mohammed1
1Department of Microbiology, Veterinary Medicine College, Wasit University, Wasit, Iraq; 2Department of Physiology and Pharmacology, College of Veterinary Medicine, Wasit University, Wasit, Iraq.
Abstract | The present study was conducted during the period from October 2013 to May 2014 in Wasit Province to detect the T. gondii in women using molecular and serological methods. Five hundred blood samples and 8 placenta specimens were collected from suspected women. The sera samples were separated and examined by ELISA to detect Toxoplasmosis serologically. In addition 89 blood samples and 8 placental tissue samples were subjected to polymerase chain reaction (PCR) technique to detect for molecular identification. The Serological results showed that 17.8% of women were positive for the private screening ELISA detects toxoplasmosis (17 % with chronic infection and 0.8% with acute infection). Moreover, it was noted that the highest rate of infection was in women who ranged in age between 20-29 years, reaching 19 .9%, but with no significant difference (P>0.05) between the ages studied. The study indicated a lack of months effect on the distribution of parasite infection rates where these different months recorded relatively close rate ranged between 14.45% - 23.07% with no significant difference (P<0.05). Regarding to polymerase chain reaction test, when a fragment of 399bp was amplified from B1 gene, the result showed that 6.74% of blood samples and 100% of placental tissue samples were positive to this test. In Conclusion the Toxoplasma infection in women was relatively high in Wasit Province.
Keywords | Toxoplasma gondii, ELISA, B1gene, PCR.
Received | December 10, 2018; Accepted | March 09, 2019; Published | June 30, 2019
*Correspondence | Sarhan Rashid Sarhan, Department of Physiology and Pharmacology, College of Veterinary Medicine, Wasit University, Wasit, Iraq; Email: [email protected]
Citation | Al-Sray AH, Sarhan SR, Mohammed HA (2019). Molecular and serological characterization of toxoplasma gondiiin women in wasit province. Adv. Anim. Vet. Sci. 7(8): 657-663.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.8.657.663
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Al-Sray et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Toxoplasmosis is a cosmopolitan zoonotic disease caused by the protozoan parasite called Toxoplasma gondii, an obligate intracellular parasite capable of infected human and all warm-blood animals (Ferguson, 2009). The parasite is mainly transmitted by food contaminated with oocyst dispersed by cats and other felines, definitive hosts, uncooked meat containing tissue cysts or unpasteurized milk containing tachyzoite stage, and transplacentally (Sukthana, 2006). T. gondii is commonly acquired in human by the oral ingestion of tissue cysts containing bradyzoites; however it can also be acquired by the ingestion of sporulated oocysts containing sporozoites that are the product of a sexual cycle in cat intestine (Tenter et al., 2000). The recognition of waterborne toxoplasmosis in humans has provided another dimension to the epidemiology of this infection (de Moura et al., 2006; Belfort-Neto et al., 2007). The diagnosis of Toxoplasmosis infection by serological test is used as common method for diagnosis of toxoplasmosis which includes sabin Feldman dye test, indirect heamagglutination test (IHT), indirect fluorescent antibody test (IFAT), complement fixation test (CFT) and intra dermal test (IDT) (Dubey., 2008). Serological tests and PCR are used in an attempt to diagnose toxoplasmosis in pregnant women (Remington et al., 2006). Burg and his colleagues First reported detection of T. gondii DNA from a single tachyzoite using the B1 gene in a polymerase chain reaction (PCR) (Burg et al., 1989). Several subsequent PCR tests have been developed using different gene targets. Overall, this technique has proven very useful in the diagnosis of clinical toxoplasmosis. Due to the importance of toxoplasmosis, so a zoonotic disease and it’s close relationship to the health of society, therefore the study was conducted in Wasit Province and its aims to detection of Toxoplasma gondii infection in human in Wasit Province by serological and PCR methods and study the effect of some risk factors such as age and months of years on the rate of infection.
MATERIAL AND METHODS
Blood Samples Collection
Five ml of blood was collected from 500 women aged between 15 to 45 old who had spontaneous abortion or suffering from problem in pregnancy during attending different AL-Kut hospitals. Samples were collected during the period from October 2013 to May 2014 and then separated and stored at -20°C until use for ELISA test. Further, 2ml of blood sample and 8 placental tissue biopsy were collected from aborted women during the third trimester. Both samples were kept at -20°C until use for DNA extraction.
Serological Testing (Elisa)
This assay was performed by using two kits (ACON, Germany) Laboratories, Inc. One was for detection of IgG and another for detection of IgM specific antibodies against T. gondii antigens in the patient’s serum.
Polymerase Chain Reaction (Pcr)
Using the primers for amplification of B1 gene in Toxoplasma gondii were designed by using NCBI- GenBank data base and primer 3 plus online and provided by Bioneer Company from South Korea (Table 1). The PCR technique was performed according to method described by (Costa and Bretagne, 2012), where the DNA extraction was performed according to the manufacturer’s, instructions (Bioneer, Korea). Briefly, 50mg of placenta tissue samples was placed in 1.5 ml microcentrifuge tube, then 200μl tissue lysis buffer and 20µl of Proteinase K were added and homogenizered by micropestle and incubated in 60°C for 1 hr. Regarding to the blood, 20μl proteinase K was added to a sterile 1.5 ml tube, and 200μl blood sample was added and mixed by vortex. After that, 200μl of binding buffer (GC) was added to each tube and mixed then all tubes were incubated at 60°C for 10 minutes.100μl of isopropanol was added to mixture and mixed well by pipetting, and then briefly spin down to get the drops clinging under the lid.
The lysate was carefully transferred into binding column (GD) (filter column) that fitted in a 2 ml collection tube, and then closed the tubes and centrifuged at 8000 rpm for 1 minute. Lysate was discarded and then 500μl washing buffer 1 (W1) was added to each binding filter column, and centrifuged at 8000 rpm for 1 minute. Washing buffer 1 and then 500μl washing buffer 2 (W2) was added to each binding filter column, and centrifuged at 8000 rpm for 1 minute. Washing buffer 2 was discarded and then the tubes were centrifuged once more at 12000 rpm for 1 minute to completely remove ethanol. After that, Binding column (GD) filter column that containing genomic DNA was transferred to sterile 1.5ml microcentrifuge tube, and then added 50μl of elution buffer and left stand the tubes for 5 minutes at room temperature until the buffer is completely absorbed into the glass filter of binding column tube. Finally, all tubes were centrifuged at 8000 rpm for 1 minute to elute DNA, the DNA extract was stored in freezer (-20°C) until using.
PCR reaction was prepared by using Accu Power PCR PreMix Kit (Bioneer, Korea) and this reaction was done according to company instructions. Then, all the PCR tubes transferred into PCR thermocycler which adjusted to conditions: initial denaturation one cycle at 95 ˚C for 5 min. then 30 cycles included denaturation 95 ˚C for 30 sec., annealing 58 ˚C for 30 sec. and extension at 72 for 40 sec. and finally final extension at 72 ˚C for 5 min.
The PCR products were analyzed by agarose gel electrophoresis used 1% agarose (Sambrook et al., 1989). The sample was considered to be positive for T. gondii DNA if the band of 399bp is observed on agarose gel, and compared with the T. gondii DNA positive control.
Table 1: The primers used to detect the B1 gene in T. gondii.
| Primer | Sequence | qPCR product size | GenBank Code no. | |
|
T. plasma gondii B1 gene |
5ˉGAACCACCAAAAATCGGAGAˉ3 | F | 399bp | 179871.1 AF |
|
3ˉGATCCTTTTGCACGGTTGT Tˉ5 |
R |
|||
Statistical Analysis
The results of present study were analyzed by SPSS program (version) software (2010), using Chi-square test and P values of p < 0.05 were considered to record statistical significance (Leech et al., 2011).
RESULTS
Detection of Toxoplasmosis Serological Examination (Elisa Test)
Incidence of Toxoplasmosis according to ELISA (IgG and IgM) test: Out of 500 serum samples from suspected cases, 89(17.8%) were positive when examined by ELISA, which indicated that 85(17%) of cases was chronic and 4(0.8%) was acute based on the mean of presence of IgG and IgM, respectively with significant differences at (p< 0.05). (Table 2)
Table 2: ELISA positive cases according to IgG and IgM test.
| ELISA test | Examined sample | Positive No. | Percentage (%) |
| IgG |
500
|
85 | 17 a |
| IgM | 4 | 0.8 b | |
|
Total |
500 | 89 | 17.8 |
Different letters refers to the significant differences at (p< 0.05).
Incidence of Toxoplasmosis according to age: Subjects was divided into four groups depending on age as illustrated in (Table 3). The results showed that the majority positive cases 19.7% were seen within 20-29 years group. Statistically results showed no significant differences at level (p<0.05).
Table 3: ELISA positive cases according to age.
| Age | Examined No. | Positive No. | Percentage (%) |
| < 20yr | 75 | 10 | 13.33 a |
|
˃ 20yr-29yr |
274 | 54 | 19.70 a |
|
˃ 30yr-39yr |
131 | 23 | 17.55 a |
| ≥ 40yr | 20 | 2 | 10 a |
|
Total |
500 | 89 | 17.8 |
Similar letters refer to the non-significant differences among ages groups at (P< 0.05).
Table 4: ELISA positive cases according to the study month.
| Month | Examined No. | Positive No. | Percentage (%) |
| October | 44 | 7 | 15.90 a |
| November | 24 | 5 | 20.83 a |
| December | 26 | 6 | 23.07 a |
| January | 83 | 12 | 14.45 a |
| February | 83 | 15 | 18.07 a |
| March | 65 | 11 | 16.92 a |
| April | 105 | 18 | 17.14 a |
| May | 70 | 15 | 21.42 a |
|
Total |
500 | 89 | 17.8 |
Similar letters refers to the non-significant differences
Incidence of Toxoplasmosis according to study months:
Accordingly, it was observed that the rates of infection during study months were relatively close, and ranged between 14.45-23.07%. Therefore, there was been no significance (P<0.05) among these percentages. (Table 4).
Molecular Examination
Eight nine blood samples that were positive for serological test were examined by conventional PCR which is showed that 6(6.74%) were positive. On the other hand, all of the eight placental tissues were collected from aborted women showed positive for PCR (Table 5).
Table 5: PCR positive cases of Toxoplasmosis
| Samples | Examined No. | Positive No. | Percentage % |
| Blood | 89 | 6 | 6.74 a |
|
Placenta |
8 | 8 | 100 b |
Different letters refers to the significant differences at (p< 0.05).
Pcr Positive Toxoplasmosis According To The Igg And Igm Test. Comparison Between Pcr And Elisa Results
Out of 85 IgG positive blood samples, only 2 were revealed positive results by PCR, while all of these 4 IgM positive blood samples were gave positive PCR result. On the other hand, all placental tissue samples which were collected from patients with positive IgG showed positive result with PCR. (Table 6) and (Figure 1).
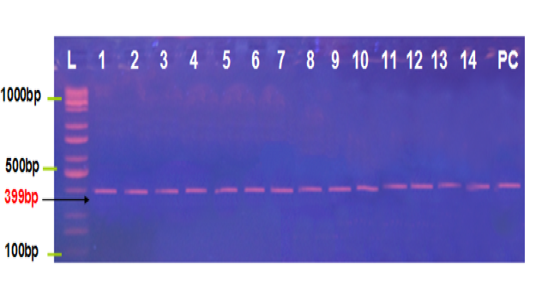
Figure 1: Agarose gel electrophoresis image that shown the PCR product analysis of B1 gene of Toxoplasma gondii at 399bp PCR product in aborted women blood samples and tissue placenta. Where L: Ladder 1000bp, lane (1-2) positive IgG blood samples, lane (3-6) positive IgM blood samples, lane (7-14) positive IgG placental tissue samples and PC positive control.
DISCUSSION
Toxoplasmosis is a zoonotic disease caused by T. gondii and has been known in many countries since 1908 (Dubey and Beattie, 1988). Author reported that the prevalence of toxoplasmosis varies among countries, depending on traditions, customs and the life styles of the inhabitants (Smith, 1991). T. gondii infection was distributed worldwide, with prevalence rates ranging from 0% to 100% in different countries and even in areas of the same State (Olivier et al., 2007). There are several methods, including immunological and molecular techniques for detection Toxoplasma infection.
Table 6: PCR positive Toxoplasmosis according to IgG and IgM test.
| Samples | Examined No. | IgG Positive | PCR Positive | % | IgM Positive | PCR Positive | % |
| Blood | 89 | 85 | 2 | 2.35 Aa | 4 | 4 | 100Ab |
| Placental tissue | 8 | 8 | 8 | 100 Ba | 0 | 0 | 0 Bb |
The capital letters refers to the vertical statistical reading while small letters refers to horizontal reading.
Different letters refers to the significant differences at (p< 0.05).
Detection of Toxicology
Serological Examination (Elisa Test): The result of ELISA examination revealed that 17.8% of suspected cases had infection with toxoplasma, so this means detection of T.gondii among women in the study area with a significant difference at (P<0.05). Among the infected cases, 0.8 % was acute cases (IgM), while 17 % was in a chronic (IgG). The low percent in IgM attributed to the IgM appears prior to after primary infection but typically does not persist, and if present, indicates acute infection; but confirmatory tests should be executed, as its specificity is not always satisfactory. By testing the avidity for T. gondii IgG, one can discriminate whether the infection was acquired recently or in the past (Montoya, 2002) as in a small percentage of the cases T. gondii IgM can remain positive for up to 24 months (Gras et al., 2004). The rate of infection in this study was nearly similar to certain studies conducted in Iraq (Mohammed and Al-Nasiry, 1996; Al-Hamdani and Mahdi, 1997; Juma and Salman, 2011) they found that the rate of infection were 20.4 %, 18.5 % and 19.17 %, respectively. While in other study conducted in Tikrit revealed a rate lower than what we are found in our work (Al-Doori, 2010) whereas higher than the rate of infection in studies of (Niazi et al., 1992) when they recorded only 8.6 % positively from eight governorates in Iraq, and in Duhok, North of Iraq, (Razzak et al., 2005) found low rate of Toxoplasma infection of about 0.97%.
The studies in neighboring countries also recorded different result contained 24 - 34% in Tehran, Iran (Noorbakhsh et al., 2002) and the seropositivity of T. gondii in a Turkish study was 1.34 % for IgM and 24.6 % for IgG (Harma et al., 2004), the seroprevalence in women was 95.5% in Kuwait (Behbehani and Al-Karmi, 1980), 37% in Jordan (Morsy and Michael, 1980), and 37.4% in Saudi Arabia (Abbas et al., 1986). In other countries the seroprevalence in women was 50% in USA (Stagno, 1980), 54% in Kenya (Griffin and Williams, 1983), 7.5% in Scotland (Jackson and Hutchinson, 1987), 37.5% in Libya (Kassem and Morsy, 1991), 47% in Nigeria (Onadeko et al., 1992), Brazil and Argentina was 11.0%, 7.3–77.5% respectively (Pappas et al., 2009). The variation in above results may be attributed to climate, cultural differences regarding hygienic, feeding habits and/or may be due to different manufacture origins of the used kits (Kortbeek et al., 2004). Related to the age and its effect on infection, the current study mentions that there is low rate of infection (10%) was seen in human with age group equal or more than 40 years (≥ 40years) while the highest infection occur in age group from 20years to 29years (˃20y-29y) with percentage (19.7%). there were no significant differences recorded, some studies results showed no significant difference with age factor but the highest infection rate occurred in age group 31-35 years (Fernands, 2010).
The results of the current study disagree with (Jassam, 2010) who recorded that the age group of 20-29 years had the lowest rate (28.6%) and the age groups of 30-39, 40-49 and ≥ 50 with the highest rates. (48.6%), (44%) and (58.1%) respectively. The results clarified that the highest percentage of infection was among the women of age group ˃ 20-29 years (19.7%), whereas the lowest was in the age group ≥ 40 years (10%) this result was coincided with (Kadihm, 2006; AL-Rawi, 2009) in Iraq, who they concluded that the main age group range of seropositive toxoplasmosis was between 20-30years. Whereas, a proven study by (Jasim, 1979) indicated that the incidence of toxoplasmosis increases with age not exceeded 50 years, also (Williams et al., 2005) pointed that the incidence increases with age, but to a peak of 34 years. This result agrees with (Al-Hindiet al., 2009) in Qatar and (Khurana et al., 2010) in Cameroon, when they confirm the highest number of infected women was in the age group 20-30years. Also the result disagreed with several results of different regions that indicated the percentage of anti-T. gondii antibodies was increased with age (Kankova et al., 2007).
High prevalence values of infection with T. gondii in our study were found in young adult women between age (20 – 29) years, this probably happened due to more frequent Toxoplasma contact in childhood and adolescence, through cats contact, soil exposure (Spalding et al., 2005).These differences between previous results and current result may be due to the differences in the specificity and sensitivity of method used for diagnosis and response of each host to the strain of parasite, the variation in parasite strains may play an important role in the stimulation of host immune response against the parasite (Suzuki and John 1994). According to the months, there is no effect on the rate of the given infections relatively close, and ranged between15-23%, this may because of the sources of infection are different in human populations and they depended on the differences in culture and eating habits (Gilot-Fromont et al., 2009), oocyst can survive for 18 months in soil, in the ethanol 95%, methanol 100%, and formalin 10% with no effectiveness its infectivity due to environmentally-resistant Oocysts are essential in the life cycle of T. gondii (Miller et al., 1972).
Molecular Examination: Molecular methods based on polymerase chain reaction (PCR) are simple, sensitive, reproducible and can be applied to all clinical samples (Bell and Ranford, 2002; Contini et al., 2005). These methods are divided into two groups. The first group consists of techniques focused on detection of T. gondii DNA in biological and clinical samples, including conventional PCR, nested PCR and real-time PCR. The second group consists of molecular methods including PCR-RFLP, microsatellite analysis and multilocus sequence typing of a single copy T. gondii DNA and those are predominantly used for strain typing (Su et al., 2010). The first protocol for molecular detection of T. gondii, for conventional PCR targeting B1gene, was developed in 1989 and has since been modified and optimized in many laboratories (Reischl et al., 2003; Switaj et al., 2005). The B1 gene, although of unknown function, is widely exploited in a number of diagnostic and epidemiological studies because of its specificity and sensitivity. There are also some studies in which the detection of T. gondii parasites was based on amplification of ITS-1 and 18S rDNA fragments, whose sensitivity was similar to the B1 molecular detection and Genotyping of T. gondii from clinical samples 105 gene (Hurtado et al,. 2001; Caldearo et al., 2006). The current study showed that the rate of infection in women according to the PCR test amounted 6.74 %, where the result was positive for out of 89 samples, two of them with chronic infection and four with acute infection examined by ELISA. The results revealed that all acute cases by ELISA (positive IgM) gave a positive result (100%) compared to the cases of chronic (positive IgG), which did not show the positive results with only two exception (2.35%).
This may be attributed to the presence of the tachyzoite in blood during the acute phase of the disease and leaving to the tissues during the chronic phase. Probably the reason for the fact that a positive result in the two chronic cases that these two cases are with subacute phase and a few number of tachyzoite still exists in the blood (Naot and Remington 1980). When placental tissue samples from seropositive (ELISA IgG positive) abortive women were examined by PCR, it was observed that all these samples were positive (100%) and this may be due to the presence of bradyzoite (tissue cyst) in placenta of the infected women in addition to the sensitivity of test in the diagnosis of infection in the event of its existence. There may be other studies reffered to the highly sensitivity of PCR test when performed to detect T. gondii in amniotic fluid, placenta and umblical cord blood (Chabber et al., 2004). Previous studies have documented that PCR can detect the parasite DNA in blood samples of women before or during pregnancy (Chabber et al., 2004). The presence of Toxoplasma DNA in maternal blood probably indicates a recent infection or apparent parasitemia, which is likely to be clinically significant (Slawska et al., 2005). PCR is the only method that can detect low levels of the T. gondii organism and even destroyed parasites (Savva et al., 1990).
The results presented by (Burg et al., 1989) showed that a single T. gondii parasite could be detected by PCR. Another explanation for the high rate of positive test results by PCR is that the amplification of B1 could represent samples containing the parasite DNA but no viable pathogens, as the PCR test does not rely on live parasites to show a positive result (Wastling et al,. 1993). By PCR technique, the study revealed that out of 8 suspected cases, 8(100%) showed positive toxoplasmosis. All these positive cases were from placenta samples. This result was nearly similar to the result of (Al-Kalaby, 2008) who recorded that 83.3% of tested samples from Iraq women was positive by PCR technique using B1 gene, while disagreed with the results of (Al- Addlan, 2007) and (Okay et al,. 2009) who reported that 17.65% and 63.49% respectively. The differences among percentages that, recorded in the current study and other studies, may be attributed to the different origins of used samples (blood, amniotic fluid, placenta tissue, etc.), and this interpretation was stated by (Marie-Francoise et al., 1999; Al-Kalaby, 2008) when they recorded a significant differences among different used samples, or due to immunocompetent or immunocompromised status of patients (Luft and Remington 1992; Israelski and Remington 1993). Conclusion Toxoplasma infection in women is relatively high in Wasit-Iraq provinces; also the prevalence of Toxoplasma infection according to ELISA Test in women was in 20-29 years old, andthere is no effect of month on the distribution of infection.
ACKNOWLEDGEMENTS
We would like to thank all hospitals staff helped us in collection of specimens.
CONFLICT OF INTEREST
This research is a personal non-profit work and there is no conflict of interest.
AUTHOR CONTRIBUTION
All authors contributed equally.
References
aborted woman in Gaza. Annals Al-Quds Med. 5: 39-47.
time PCR-based assay using the lightcycler system for detection of T. gondii bradyzoite genes in blood specimens from patients with toxoplasmic retinochoroiditis. Int. J. Parasitol. 35: 275–283.
toxoplasmosis. M. Sc. Thesis. College of science. University of Baghdad. pp130 (in Arabic)
Toxoplasma have moresons. Natur Wissen Schaften. 94: 122-127.






