Advances in Animal and Veterinary Sciences
Research Article
Biofilmicidal Efficacy of Five Disinfectants against Campylobacter jejuni on Different Poultry Farm Surfaces
Samah E. Laban1*, Mohamed M. Hamoud2
1Department of Veterinary Hygiene and Management, Faculty of Veterinary Medicine, Cairo University. Giza, 12211, Egypt; 2Department of Poultry and Rabbit diseases, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt.
Abstract | Biofilm formation is a complex process, consists of a cord of molecular and physiological events that take place through several stages including adherence, formation of microcolonies, tridimensional structuring, and maturation. Biofilmacts as a reservoir not only for food production facilities but also for animal and poultry husbandry environment and considers a source of unceasing supplier of zoonotic and foodborne microorganisms infecting both hosts and consumers. Among these organisms, Campylobacter jejuni (C. jejuni) colonizes in bird hindgut with high counts. C. jejuni is widely considered as a main cause of gastroenteritis in poultry products consumers and has the ability to form biofilm on surfaces inside poultry farms and slaughterhouses or food contact surfaces. Previous studies detected the biofilm formation and efficacy of sanitizers on food contact surfaces, but fewer examined within and or on the farm components. This study aims to study the ability of C. jejuni to form biofilm on plastic, galvanized wire and concrete surfaces as simulating in poultry farms and evaluate the efficacy of five commercial disinfectants; Pyam®, Klorsept®, Calcium hypochlorite®, PronTech® and Virukill®. All which used at two concentrations 0.5% and 1%. The used surfaces showed biofilm formation by different degrees. Our results revealed that Pyam®, Klorsept® and Ca. hypochlorite® showed the highest logarithmic reductions of biofilm count followed by potassium peroxymonosulfate and halide NaCl combination Virukill® while the lowest reductions were obtained using QAC (Alkyl Dimethyl Benzyl Ammonium Chloride 40% with 60 % Urea, PronTech®). In conclusions, the use of efficient disinfectants reduces the risk of C. jejuni infections through direct and indirect routes of infection.
Keywords | Biofilm, Campylobacter jejuni, Surface material, Disinfectants, Logarithmic reductions.
Received | January 02, 2019; Accepted | May 08, 2019; Published | June 15, 2019
*Correspondence | Samah E Laban, 1Department of Veterinary Hygiene and Management, Faculty of Veterinary Medicine, Cairo University. Giza, 12211, Egypt; Email: [email protected]
Citation | Laban SE, Hamoud MM (2019). Biofilmicidal efficacy of five disinfectants against campylobacter jejuni on different poultry farm surfaces. Adv. Anim. Vet. Sci. 7(8): 634-640.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.8.634.640
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Laban and Hamoud. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Biofilm is a firm attachment of bacterial aggregates on an abiotic or living surface embedded in a self-produced matrix of extracellular polymeric substances and becomes irreversibly associated with this surface and can’t be removed by gentle rinsing (Donlan, 2002). Under protection conditions, the bacterial community can provide longer period of survival, resistance to stressful environmental conditions and antimicrobial agents which used for sanitation representing constant source of contamination for production systems (Trachoo & Brooks, 2005). Therefore, properties of contact surfaces have a strong influence on the adherence of microorganism. Generally, bacterial adhesion affected by hydrophobicity, degree of surface roughness, presence of organic and inorganic compounds, flow conditions either static or dynamic and presence of antimicrobial agents (Donlan & Costerton, 2002, Sousa et al., 2009). Thus, biofilm prevention and elimination will require a combination of physical, chemical and biological methods (Malaeb et al., 2013). Many studies evaluated the degree of different antimicrobials efficacy against biofilm especially their mode of action which found to influence their ability to transfer through biofilms. Biofilm matrix has a polyanionic nature that may increase resistance of biofilm to antimicrobial agents (Cloete, 2003). Chemical reaction of antimicrobials with biofilm matrix might rely on their interaction with deep biofilm layers that bears higher resistant bacteria (Anderl et al., 2000).
Campylobacter species are widely distributed in most warm-blooded animals. Poultry is considered the main reservoir probably due to their higher body temperature (Skirrow, 1977). Once Campylobacter gain entrance to a poultry flock even with low counts as low as 40 colony forming unit (CFU), it can spread rapidly and colonized with high numbers in bird cecum followed by shedding up to 109 Campylobacter/g fecal contents (Cawthraw et al.,1996; Newell & Fearnley, 2003). Likewise, in poultry slaughterhouses during processing and evisceration ,intestinal rupture might be happened and triggered high carcass contamination along with the processing line (EFSA, 2010; Wagenaar et al., 2013). Thus, reducing the prevalence of Campylobacter colonization in living birds will decrease the introduction of high numbers of Campylobacter to the slaughterhouse and environment (Wagenaar et al., 2013). The rapid spread of Campylobacter inside the flock is mainly due to horizontal transmission through water and other risk factors in thepoultry environment including; poor cleaning and sanitation, poor biosecurity, poor maintenance, short empty periods and insects and wild animals beside the presence of persistent reservoir such as biofilm (Shreeve et al., 2002; Hanning, 2008).
Little information was available concerning Campylobacter as a biofilm constituent while some studies found that C.jejuni maintains its viability in low nutrient media and normal atmospheric conditions and could be isolated from water, watering system (pipes, nipples and drinkers) and poultry houses that can then detached from the biofilm to induce infection (Costerton et al., 1994; Trachoo et al., 2002). Unlike other enterobacteria, Campylobacter prefers static conditions for fixation on surfaces as its flagellar motility plays a key role in migration and microcolonies formation after 4 hours from adhesion followed by fixation and biofilm formation (Sulaeman et al., 2012; Theoret et al., 2012). Maturation requires about three days then dispersion start to occur (Cappitelli et al., 2014; Rossi et al., 2017). Also, some extrinsic factors can act directly on Campylobacter adhesion capacity like surface type (Moe et al., 2010).
Routine house cleaning and disinfection is suggested to be adequate for C. jejuni decontamination. However, in houses where no cleaning and disinfection between two frequent flocks applied, decontamination can also occur due to poor environmental conditions in absence of birds. Previous studies reported that the empty period following a positive flock should be 14 days which is enough time to reduce the residual bacterial contamination in and around previously infected houses (Hald et al., 2000; Wedderkopp et al., 2000; Hiett et al., 2002). Other studies reported that Campylobacter count increased up to 250-fold when exposed to surfaces carrying previously formed biofilm and they claimed that to the ability of biofilm matrix to increase bacterial attachment, increased the resistance of Campylobacter to disinfectants and cleared the need for frequent cleaning of surfaces before disinfection to reduce C. jejuni reservoirs (Trachoo & Frank 2002). So, removal of biofilm needs extensive mechanical action in scrubbing surfaces with brushes together with using cleaning agents before disinfection to increase the disinfectants efficiency while hard brushing should be avoided to circumvent abasing and scratching surfaces that help biofilm formation (Bremer et al., 2002). Also, frequent disinfection particularly of quaternary ammonium compounds (QACs) might contribute to development of disinfectant resistant microorganisms (Langsrud et al., 2003).
Inthe present study, we declare the ability of C.jejuni to form biofilm on materials resembling those that present in poultryfarmsas concrete, galvanized wire, and plastic. After recognition of biofilm formation five commercial disinfectants commonly used for poultry farm disinfection were examined to detect their biofilmicidal efficacy against C. jejuni.
MATERIALS AND METHODS
Bacterial Strain Preparation and Test Surfaces
Well identified Campylobacter jejuni strain was kindly provided by the Microbiology unit, Animal Health Research Institute (AHRI), Dokki, Giza, Egypt and used in the current study. The strain was cultured on CCDA agar (Campylobacter Blood-Free Selective Agar Base) for 48 h at 42oC followed by overnight growth in BHI broth at 42oC under microaerobic conditions using Campy Gen 2.5 l, Oxoid. Overnight culture was gently shaken for 30 sec then the bacterial count was adjusted to 0.5 McFarland that contain about 108 CFU/ml (Brown et al., 2014). Coupons of plastic, concrete and galvanized wire were used to build biofilm. Before conducting the experiment, coupons were treated using 70% ethanol then thoroughly rinsed with distilled water to detach any soil followed by autoclaving at 121 °C for 15 minutes prior to use (Hoa et al., 2015).
Biofilm Growth, Detection of Biofilm Formation and Cell Enumeration
After preparation of the bacterial suspension, the coupons were immersed and incubated at 37 °C for 72 h under microaerobic conditions (Teh et al., 2010). At the end of incubation periods, coupons were rinsed three times using distilled water for removal of planktonic bacteria and air-dried biofilms were stained using 0.1% (w/v) crystal violet at 28°C for 20 min (Tang et al., 2012).
Biofilm cells were enumerated according to the method described by Hao et al. (2016) to determine the initial bacterial count.In brief; coupons were aseptically withdrawn from the strain culture and placed separately in a sterile glass petri dish then rinsed twice in 10 mL of distilled water to remove non-attached bacteria (Rochex & Lebeault, 2007). Cells attached to each coupon were detached by swab method; both sides of each coupon were thoroughly swabbed using a pair of cotton swabs previously soaked in sterile saline solution to detach as many cells as possible from the surfaces. The heads of the cotton swabs were broken off and received into tubes containing 5ml sterile saline. The bacteria present on swabs were re-suspended by manual shaking for 30 s for detachment of bacteria. Cell suspension was then serially diluted, a volume of 0.1 mL of each dilution was plated on CCDA in duplicates and the plates were incubated at 42 °C for 48 h. After incubation, typical colonies representing C. jejuni were counted (Teh et al., 2010).
Biofilmicidal Efficacy of Commercial Disinfectats
Five commercial disinfectant were used to evaluate their biofilmicidal efficacy against C.jejuni biofilm cells as shown in Table 1. Disinfection was carried out at room temperature for a contact time of 5 minutes. After biofilm formation, coupons were aseptically withdrawn from the strain culture, rinsed twice in 10 mL of distilled water to remove non-attached bacteria then separately exposed to 2 ml of test disinfectant for the given time at room temperature. Coupons were then swabbed using two moistened sterile cotton swabs, received in a tube containing 5 ml of disinfectant neutralizer solution consisting of 3% Tween 80, 0.3% Lethcine, 1% Histidine, 0.5% Sodium thiosulphate and 3% Saponine (Douglas & Kampf, 2010). The number of bacteria left after disinfection is enumerated as mentioned earlier.
RESULTS AND DISCUSSION
Our results revealedthe ability of C. jejuni to form a biofilm on three surfaces commonly present in poultry farms; plastic which represents drinker and plastic cover, galvanized wire which could be feeder, cage or even perch and concrete which represents wall and floor as shown in Tables 2 and 3 at which show biofilm development after 3 days on all used surface types, but the bacterial count within the biofilm was the highest on concrete (log 6.7) followed by galvanized wire (log 5.5) while plastic wasthe least (log 4.9). This difference in degree of bacterial adherence and biofilm formation may be correlated to the hydrophobicity of surface, the degree of its roughness or to surface wear roughness and the flow conditions either static or dynamic (Verran & Boyd., 2009). In contrast, previous studies revealed that salmonella biofilm formation with high density was on plastic, followed by concrete and galvanized wire (Joseph et al., 2001) while Others concluded that following the bacterial attachment, the numbers of bacteria adhered to plastic may not be increased (Beresford et al., 2001). Our results declare the biofilmicidal efficiency of five commercial disinfectants using two different concentrations 0.5% and 1% against C. jejuni after 3-day from the biofilm formation which previously formed on the tested coupons.
Table 1: Disinfectants Used for Biofilmicidal Efficacy Evaluation against C. jejuni
| Disinfectant | Active principle | Manufacturer |
|
Pyam® |
Sodium Dichloroisocyanurate (NaDCC) | Laboratory Pyam SA (Argentina) |
|
Klorsept® |
Sodium Dichloroisocyanurate (NaDCC) |
Medentech (Ireland) |
|
Calcium hypochlorite® |
Calcium hypochlorite 89% | Egyptian company for chemicals production (Egypt) |
|
PronTech® |
Alkyl Dimethyl Benzyl Ammonium Chloride 40%, Urea 60% |
United Promotions INC (Atlanta, USA) |
|
Virukill® |
Potassium peroxymonosulfate 50%, NaCl 3% |
UBM (Egypt) |
For plastic coupons results showed that all the used disinfectants at both concentrations could achieve total reduction of C. jejuni except PronTech® failed at 0.5% concentration and achieved log reduction 26.5% from the initial count (Table 2 and 3; Figure 1A,B). Regarding galvanized wire coupons, results showed that Pyam® and PronTech® failed to achieve complete reduction of the bacteria withlog reduction 51% and 40%, respectively (Tables 2, 3 and Figure 1A,B). However, the results of concrete coupons were completely different which were the lowest efficacy obtained for disinfectants. Only Pyam® 1% and Calcium hypochlorite® 1% could completely reduce C. jejuni biofilm on concrete coupons while other disinfectants gave variable reductions with log reductions 55% for Pyam® 0.5%, 37% for Klorsept® 0.5% and 75% for Pyam® 1%, 51% for Calcium hypochlorite® 0.5%, 37% and 40% for Virkukill®
Table 2: Biofilmicidal Efficacy of Disinfectants Against C. jejuni on Different Surfaces
| Surface type | Initial log | Disinfectant | |||||||||
|
Pyam® |
Klorsept® |
Calcium hypochlorite® |
PronTech® |
Virukill® |
|||||||
| 0.5% | 1% | 0.5% | 1% | 0.5% | 1% | 0.5% | 1% | 0.5% | 1% | ||
| Plastic | 4.9 | 0 | 0 | 0 | 0 | 0 | 0 | 3.6 | 0 | 0 | 0 |
| Galvanized wire | 5.5 | 2.7 | 0 | 0 | 0 | 0 | 0 | 3.3 | 0 | 0 | 0 |
| Concrete | 6.7 | 3 | 0 | 4.2 | 1.7 | 3.3 | 0 | 4.7 | 4.4 | 4.2 |
4 |
Table 3: Log. Reduction Percentage of Biofilm after Disinfectant Treatmenton Different Surfaces after 5 minutes
| Surface |
Pyam® |
Klorsept® |
Calcium hypochlorite® |
PronTech® |
Virukill® |
|||||
| 0.5% | 1% | 0.5% | 1% | 0.5% | 1% | 0.5% | 1% | 0.5% | 1% | |
| Plastic | 100% | 100% | 100% | 100% | 100% | 100% | 26.5% | 100% | 100% | 100% |
| Galvanized wire | 51% | 100% | 100% | 100% | 100% | 100% | 40% | 100% | 100% | 100% |
| Concrete | 55% | 100% | 37% | 75% | 51% | 100% | 30% | 34% | 37% |
40% |
(Figure 1A,B). The lowest log reduction was obtained from PronTech® (QACs) that were 30% and 34% using 0.5% and 1%, respectively (Figure 1A,B).
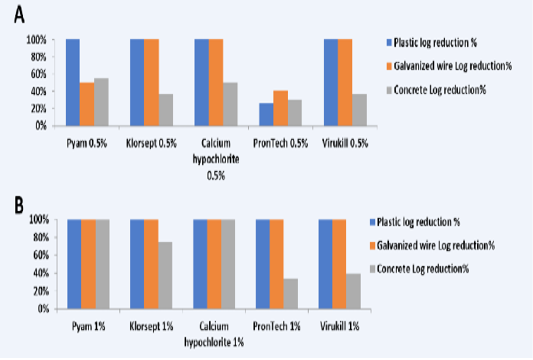
Figure 1: Log reduction % of used five disinfectants at concentration A) 0.5% and B) on different surfaces (plastic, galvanized wire and concrete)
Our results in this study showed that disinfectants containing chlorine releasing agents; Pyam® and Klorsept® and Calcium hypochlorite® were more effective in reducing C. jejuni biofilm cells count on the three types of surfaces >3 log reductions equals to 99.9% count reduction which is the target for achieving effective inactivation of attached bacterial biofilm (Aarnisola et al., 2000; Frank & Chmielewski., 1997, Mettler & Carpentier, 1999). Lower efficacy obtained from the fourth chlorine releasing agent containing Potassium peroxymonosulfate and halide NaCl (Virukill®), however, the lowest log reduction was obtained by the QAC with urea (pronTech®) (Table 2 and 3). Chlorine based agents are the most broadly used disinfectants as they are highly active oxidizing agents whose activity depend on hypochlorite formation which acts by oxidative activity of proteins and systems of essential cell enzymes (McDonnell & Russell, 1999; Wirtanen et al., 2001). Previous studies correlate the efficacy of antimicrobials to their molecule size and diffusion ability, at which small sized hypochlorite molecules known for removing exopolysaccharides from the surface which prevent the adherence of new bacteria (Meyer, 2003; Stewart, 2003).
Forbiofilms of C. jejuni as a single component, Trachoo & Frank, (2002) found that it was sensitive to as low as 50 ppm chlorine and QACs after 45 seconds which was the same concentration failed after 180 seconds to inactivate C.jejuni in mixed biofilms indicating that chlorine was the most effective sanitizers however QACs showed lower efficacy and required longer time to achieve the required reduction rate. Rossoni and Gaylarde, (2000) demonstrated that high reductions in the number of adhered cells of E. coli, S. aureus and P. fluorescens on stainless steel surface with hypochlorite at 100 or 200 ppm. Likewise, Caixeta et al. (2012) found that sodium dichloroisocianurate was the most efficient sanitizer in reducing adhesion and biofilm by P. aeruginosa at 7 and 28 °C. Also, Ahmed, (2017)found that the logarithmic reduction percentages of sod. hypochlorite, Ca.hypochlorite and Virkon at 1% concentration on galvanized wire coupons were 100%, 100%, 51% for Salmonella typhimurium biofilm and 100%, 100%, 41% for Pseudomonas aeruginosa biofilm, respectively. Somers & Lee-Wong, (2004) determined the efficacy of two cleaning and sanitizing combinations on Listeria monocytogenes biofilms on different surfaces, which revealed that 3-log reduction was achieved for all surfaces including plastics and bricks following hypochlorite application.
QACs with urea (PronTech®) showed the lowest log reductions on all surfaces as disinfection using QACs can lead to emergence of disinfectant resistant microorganisms (Langsrud et al., 2003). The mode of action of anti-microbial agents along with the molecule size can influence on molecule transfer through biofilms. QACs act through adsorbtion to cell wall then reacts with the cytoplasmic membrane resulting in release of intracellular constituents (Trueba et al., 2013). QACs are positively charged big molecules, having strong affinity to protein and lipids which lead to difficulty in diffusion through the matrix before reaching the deep biofilm layers (Smirnova et al., 2010).Previous reports showed that biofilmicidal efficacy on B. cereus caused by QACs were ~50% and their ability to remove biofilm were around 15% as penetration of biofilm is not necessarily accompanied with killing the embedded cells (Araújo et al., 2014). Beauchamp et al. (2012) reported that quaternary ammonium chloride compounds were the least effective together with hypochlorite against E. coli O157:H7 biofilm cells, regardless of biofilm age, sanitizer concentration or exposure time however potassium peroxymonosulfate have had better efficacy
CONCLUSIONS
C. jejuni biofilms buildup in poultry environment at different degrees regardless to time elapsed, biofilm on concrete was higher than galvanized wire and that of plastic.C. jejuni biofilms were sensitive with variant degrees to test disinfectants. Disinfectants containing chlorine releasing agents showed higher reductions than those have QACs with urea. Disinfectant efficacy seemed to be affected bythe type of contact surface, concrete showed lowest reductions in C. jejuni biofilm counts after disinfection treatment.
ACKNOWLEDGEMENTS
We would like to thank all co-workers and colleagues in the Department of Animal Hygiene and Veterinary Management and Department of Poultry and Rabbit diseases, Faculty of Veterinary Medicine, Cairo University Egypt for their technical support. Special thanks to Prof Osama K Zahran, Faculty of Veterinary Medicine, Cairo University for his help and support during work and writing this manuscript.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interest.
AUTHORS CONTRIBUTION
All authors contributed equally.
REFERENCES






