Advances in Animal and Veterinary Sciences
Research Article
A Comparison Between Autogenous Skin Graft and Alloskin Graft in Dogs
M.A. Ahmed, Layth M. Alkattan*
College of Veterinary Medicine, Department of Surgery and Theriogenology Mosul, Iraq.
Abstract | This study was intended to compare the autogenous skin graft and alloskin graft in twelve adult local breed dogs. Animals were allocated randomly into two equal groups, 6 animals each. A 10cm circular skin pieces, full thickness, were harvested aseptically from the lower abdomen. These grafts were prepared for grafting. The created defects were repaired with the prepared grafts and were fixed firmly with an underlying bed of the same animal. Postoperatively, both macroscopic and microscopic examinations were performed at the periods of a week, a month and two months). The same procedure was followed in the second group, except the skin graft was obtained from a dog and fixed to an induced defect in another dog. In the first group, results did not exhibit any troubles in the physiological status or side effects during the first-weekpost-grafting. Histopathological examination revealed signs of inflammatory reaction, necrosis, and degeneration of the dermis layer. Two months later, the formation of granulation tissue (scar) and restoration of normal skin color was observed without hair follicles formation. Histologically, there was an extension of collagen fibers at the site of operation. However, in the second group, signs of inflammation and hemorrhage were detected after a week of the operation. Histological examination revealed the absence of signs of rejection, little hair formation and pigmentation similar to the donor skin. Two months postoperatively, there was sloughing of the epidermis layer of the grafted tissue. Histologically, there was deposition of collagen fibers (sheet-like) as a response to granulation tissue formation. To sum up, the study specified the successful outcomes of using skin allograft where the site of operation renewed with the same color of donor skin with a little hair formation.
Keywords | Autograft, Allograft, Skin, Dogs
Received | October 15, 2018; Accepted | January 14, 2019; Published | May 03, 2019
*Correspondence | Layth M Alkattan, College of Veterinary Medicine, Department of Surgery and Theriogenology Mosul, Iraq; Email: [email protected]
Citation | Ahmed MA, Alkattan LM (2019). A comparison between autogenous skin graft and alloskin graft in dogs. Adv. Anim. Vet. Sci. 7(6): 516-521.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.6.516.521
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Ahmed and Alkattan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Skin wounds are the most common injuries seen in the veterinary field this may handle either by primary closure with many kinds of suture materials which can be used effectively for wound closure or by skin grafting this necessary to achieve skin closure with minimum side effects and good outcome (Micheal and Llewellyn, 1983). Commonly, the term “graft” refers to either an allograft or an autograft. Autograft is a type of grafting that uses skin from an area of the body to repair damage in another area,but there has to be enough undamaged skin available and the patient has to be healthy (Revis, and Michael, 2002). Skin grafts are an excellent modality used to reconstruct wound located on the limbs and other areas of the body. It has been used for over a century to resurface superficial defects of many kinds of these types the auto skin graft, which was used to repair the injury or area that has lost tissue that too big to sew the edges together directly (Aper and Smeak, 2003). Auto skin graft became a widely used method of treating organ loss. For instance, it is an effective procedure for the short-term treatment of full-thickness skin loss, especially meshed skin in severely burned animals. (Machensa et al., 2000). Survival of a free meshed skin graft is dependent on adherence, plasmatic imbibition, and revascularization. Adherence is the process by which the skin graft adheres to the underlying wound defect through the development of fibrinous then fibrous adhesions between the skin graft and wound bed (Tong and Simpson, 2012). For better graft immobilization, the use of vacuum-assisted closure devices following the surgery of skin grafting has been reported in dogs. Vacuum-assisted closure devices can improve skin graft survival rates through better adherence, plasmatic imbibition and possibly revascularizations (Julius, 2012). Improvement of dermal and epidermal recovery is a critical objective for the treatment of most types of wounds (Cabeza-Martinez et al., 2006). With extensive full-thickness skin loss exceeding 30% of the body surface, autografts are often not available in sufficient quantities (Auger et al., 2009). Temporary coverage can be obtained by skin allograft from volunteers, diseased free cadavers, or the patient’s relatives and friends and is often a life-saving measure. It is using as a biological dressing that can assist in several functions. Firstly, to provide clean granulating area prior to auto-grafting; secondly, to protect the open wound from protein and water loss until auto-grafting is available; thirdly, to minimize bacterial count and pain at the site of an open wound. Skin allograft has also been recommended for protecting the vital organs insecond-degree burns (Marcia, 2015). Contra-indications to skin grafting can be defined as local and systemic factors. Local factors contain recipient site infection and limited blood supply (presence of cartilage or tendon, or radiated tissue). Systemic factors that compromise skin grafting include anemia, cachexia, anorexia, chronic inflammatory conditions, uremia, hypo-perfusion, and infection. (Marcia, 2015). The objective of the present study was to compare between using auto-skin graft and alloskin graft for repairing a large skin defect in dogs.
Materials and methods
Twelve healthy local breed dogs of both sexes were included in the present study. Animals aged between 12-18 months and weight around 20-22 kg. The experimental animals kept at the same environmental and feeding conditions. Animals were allocated in two equal groups, six dogs each. Animals in the first group were prepared for the routine surgical procedure of skin grafting. Protocol of anesthesia included the administration a mixture of Xylazine\ Ketamine at 30 mg IM, respectively for induction and maintenance of anesthesia. A circular piece of skin with 10 diameter was harvested aseptically as the full thickness from the lower abdomen. This graft was prepared for grafting so all fatty, underlying tissue was removed, and small holes were made at the skin graft to allow fluid drainage. At same time skin defect as, circular shaped 10 cm diameter was induced at the lateral aspect of the forelimb. This defect was reconstructed with the same skin graft that harvested from the lower abdomen. The graft attached firmly with adjacent tissue and fixed with many stitchesat a different site to make it more adherent to the adjacent tissue. Macroscopic and histopathological examinations were utilized to confirm the diagnosis; biopsies were taken aseptically at different periods as (one week, one month and two months). In the second group, similar steps were followed as in the first group, except the graft was taken from dogs and implanted in another dog of same species. At each one week and one month, tissue samples were taken from the junction of normal and grafted tissue, fixed in 10% neutral buffered formalin for 72 hours, then histopathological examination was utilized as described by (Luna, 1968). Tissue sections were stained by Harri’s hematoxylin and alcoholic eosin (Suvarna et al., 2013). Later the stained slides examined at 40X, 100X, and 400X powers.
Results
All operative animals involved in this study were survived at both the initial surgical procedure and the intended study period. There were no troubles in the physiological status or any side effects associated with anesthesia during the study period. The protocol of anesthesia was enough to complete the operation. One-week post-grafting, animals in the first group exhibited cardinal signs of inflammatory reaction included swelling and redness (due to hemorrhage) with an area of necrosis and sloughing of the dermis layer (Figure 1). Histopathological observations showed two sides of the tissue, the original tissue showed no signs of rejection (A), while the grafted tissue (B) showed necrosis and degeneration of the dermis layer (Figure 2). After one month the results exhibited formation of granulation tissue (scar) with the restoration of normal skin color without hair follicles formation (Figure 3). Histopathological results exhibited an extension of collagen fibers to connect the adherent sides or surface of the original tissue (A) and grafted tissue (B) (Figure 4). Showed grafted tissue (A) with the restoration of the epidermislayer and formation of the spongiosum layer. (Figure 5) there was no hair growth at the surface of the graft (Figure 3). Two months post-grafting the results exhibited formation of granulation tissue (scar) with the restoration of normal skin coloration without hair formation (Figure 6). Histopathological examination demonstrated the original tissue (A) and grafted tissue (B) where the main differences between two sides of tissue were the presence of hair follicle and sweat glands Figure 7. The results of the second group grossly showed inflammatory signs, areas of hemorrhage, necrosis, and skin ulcers first-week postoperatively (Figure 8). Histopathologically showed original tissue (A) and grafted tissue (B). Necrosis and sloughing of the epidermis layer of grafted tissue (Figure 9) showed an acute inflammatory reaction (arrowhead) under the grafted tissue (Figure 10). After a month grossly showed granulation tissue formation with absent of gross signs of tissue rejection (Figure 10). Histological results show the original tissue (A) and grafted tissue (B). Necrosis and hemorrhage present in epidermis layer of grafted tissue (Figure 11). Two-months post grafting showed absent signs of rejection little hair formation and pigmentation like the donor skin (Figure 12), Histopathologically Showed original tissue (A) and grafted tissue (B), also granulation tissue formation in grafted tissue Figure 13, deposition of collagen fibers as sheets
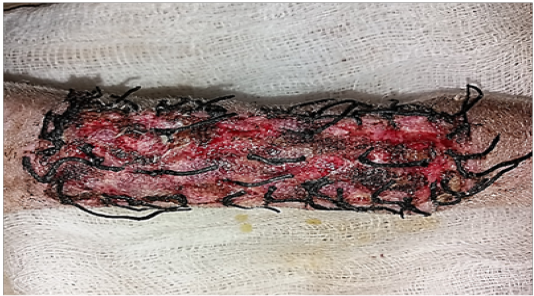
Figure 1: Gross image of autograft site of dog’s leg after one week of surgery. Showed cardinal signs of inflammatory reaction included swelling and redness (due to hemorrhage) with an area of necrosis and sloughing of the dermis layer.
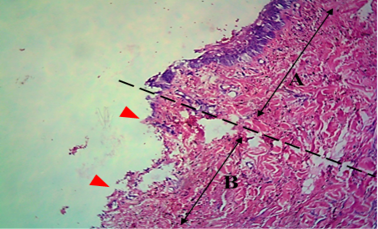
Figure 2: Histological section of autograft site of dog’s leg after one week of surgery. Showed two sides of the tissue, the original tissue showed no signs of rejection (A), while the grafted tissue (B) showed necrosis and degeneration of the dermis layer (arrowhead). H&E, 100x.
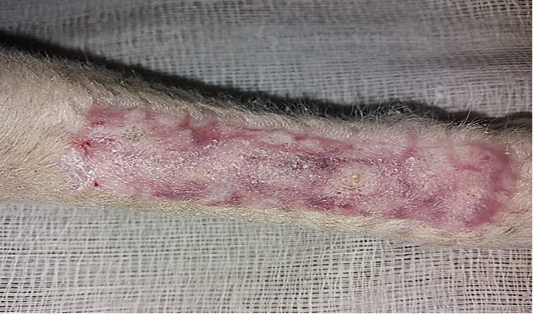 Figure 3: Gross image of autograft site of dog’s leg after one month of surgery. Showed formation of granulation tissue (scar) with the restoration of normal skin color without hair formation.
Figure 3: Gross image of autograft site of dog’s leg after one month of surgery. Showed formation of granulation tissue (scar) with the restoration of normal skin color without hair formation.
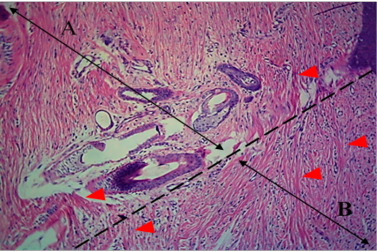
Figure 4: Histological section of autograft site of dog’s leg after one month of surgery. Showed original tissue (A) and grafted tissue (B). Extension of collagen fiber to connect the wound sides (arrowhead). H&E, 100x.
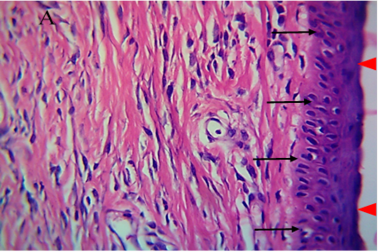
Figure 5: Histological section of autograft site of dog’s leg after one month of surgery. Showed grafted tissue (A) with the restoration of the epidermis layer (arrowhead) and formation of spongiosum layer (arrow).
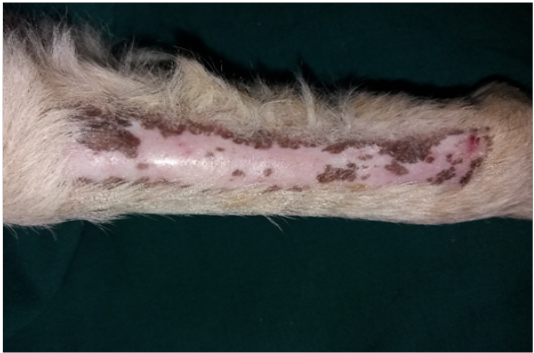 Figure 6: Gross image of autograft site of dog’s leg after two months of surgery. Showed formation of granulation tissue (scar) with the restoration of normal skin color without hair formation.
Figure 6: Gross image of autograft site of dog’s leg after two months of surgery. Showed formation of granulation tissue (scar) with the restoration of normal skin color without hair formation.
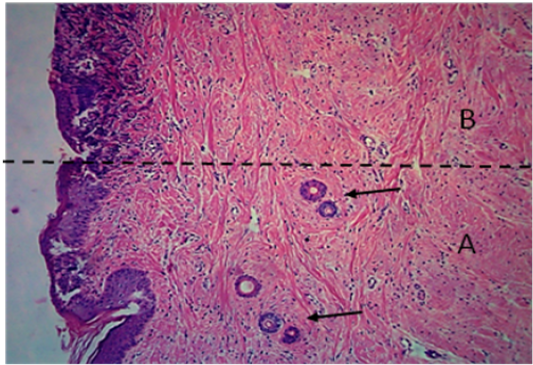
Figure 7: Histological section of autograft site of dog’s leg after two months of surgery. Showed original tissue (A) and grafted tissue (B), the main differences between the two sides of tissue was the presence of hair follicle and sweat glands (arrow). H&E, 100x.
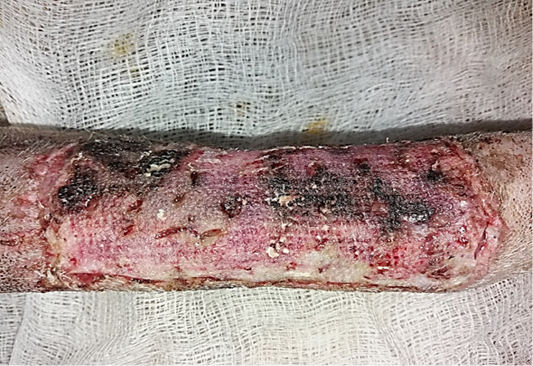 Figure 8: Gross image of allograft site of dog’s leg after a week of surgery. Showed inflammatory signs, areas of hemorrhage, necrosis and skin ulcers.
Figure 8: Gross image of allograft site of dog’s leg after a week of surgery. Showed inflammatory signs, areas of hemorrhage, necrosis and skin ulcers.
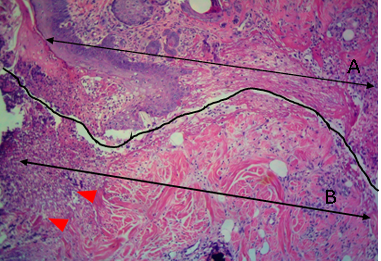 Figure 9: Histological section of allograft site of dog’s leg after a week of surgery. Showed original tissue (A) and grafted tissue (B). Necrosis and sloughing of the epidermis layer of grafted tissue (arrowhead). H&E, 100x.
Figure 9: Histological section of allograft site of dog’s leg after a week of surgery. Showed original tissue (A) and grafted tissue (B). Necrosis and sloughing of the epidermis layer of grafted tissue (arrowhead). H&E, 100x.
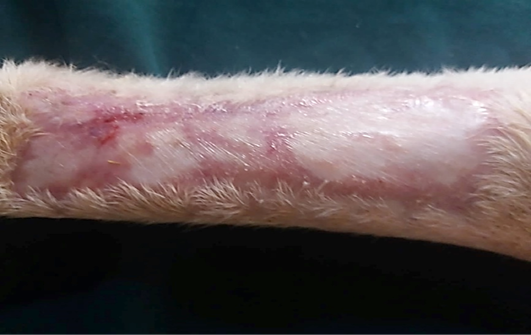 Figure 10: Gross image of allograft site of dog’s leg after a month of surgery. Showed granulation tissue formation with the absent of gross signs of tissue rejection.
Figure 10: Gross image of allograft site of dog’s leg after a month of surgery. Showed granulation tissue formation with the absent of gross signs of tissue rejection.
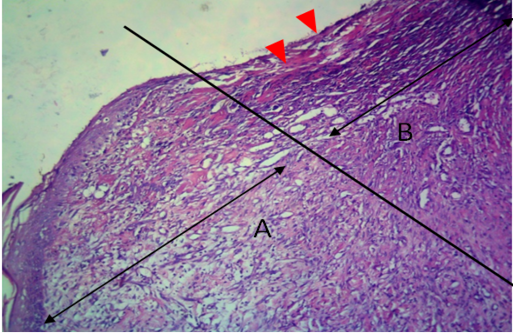 Figure 11: Histological section of allograft site of dog’s leg after one month of surgery. Showed original tissue (A) and grafted tissue (B). Necrosis and hemorrhage present in epidermis layer of grafted tissue (arrowhead). H&E, 100x.
Figure 11: Histological section of allograft site of dog’s leg after one month of surgery. Showed original tissue (A) and grafted tissue (B). Necrosis and hemorrhage present in epidermis layer of grafted tissue (arrowhead). H&E, 100x.
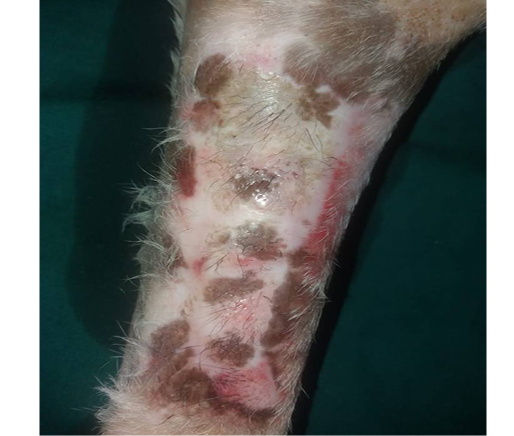 Figure 12: Two-months post grafting showed absent signs of rejection little hair formation and pigmentation like the donor skin
Figure 12: Two-months post grafting showed absent signs of rejection little hair formation and pigmentation like the donor skin
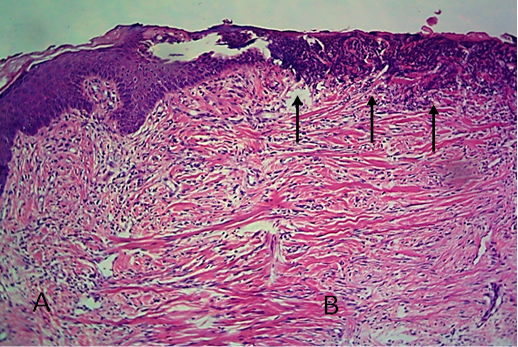 Figure 13: Histological section of allograft site of dog’s leg after two months of surgery. Showed original tissue (A) and grafted tissue (B), also granulation tissue formation in grafted tissue.
Figure 13: Histological section of allograft site of dog’s leg after two months of surgery. Showed original tissue (A) and grafted tissue (B), also granulation tissue formation in grafted tissue.
Discussion
Skin grafts are vital tissues which serve as a source for reconstructive surgery. Tissue grafts should be obtained as the full thickness from a donor site to repair the excisional big tumor if the primary closure is not possible and severe skin loss occurs (Darwish, 2011). The removal of subcutaneous fat during graft preparation is essential to ensure graft viability but can be time-consuming, tedious and incomplete (Ownsend et al., 2012). Meshing the graft by making multiple staggered incisions, give advantages of further increase drainage capabilities and increases the size of the graft (Marcia, 2015). In the present study, during the first few days post operation, we did not record any problems in all treated animals. Animals were survived along of operations periods. In the first two days, the graft appeared with a white color due to local vasoconstriction. Then during the first week, the site of grafting appeared purplish or cyanotic, this might result in hemoglobin breakdown, these findings were also recorded in a previous work. (Marcia, 2015).
In our study, revascularization occurs especially in the first group and the graft appeared more adherent to the wound bed during the first few days. This may be attributed to the direct connection of local graft and wound vessels and inoculation. Survival of an implanted skingraft is dependent on the degree of adherence and revascularization between donor and recipienthost. The adhesion occurs due to complete adherence of graft with wound bed by developing of fibrinous followed by fibrous tissue formation, this finding is consistent with (Tong and Simpson, 2012). Nutrition is achieved by diffusion of nutrients from the fluid layer between the graft and wound bed (plasmatic imbibition). (Marcia, 2015). In addition, through the extension of blood vessels from the wound into the skin graft and/or ingrowth of host vessels into existing endothelial channels; these results might be obtained due to good and firm fixation of skin graft and adequate immobilization and bandaging. The inadequate bandaging and movement of skin graft are the most common causes of skin graft failure. In such condition, this allows the development of a hematoma between the graft and wound bed, which prevents adherence and plasmatic imbibition of the skin graft, and movement of the skin graft, which prevents revascularizations of the skin graft (Tong and Simpson, 2012). There was poorly growth of hair follicles and hair in both in both group and the donor area was healed as an opened wound, a similar observation was stated in a previous work where the epithelialization occurs at the grafted area leading to little or absence of hair formation (Keever and Barden, 1978).
The result of the histopathological examination revealed absence signs of tissue rejection reaction in both allograft and autograft tissues, this associated with restoring normal tissue architecture of wound healing, represented by the formation of granulation tissue and angiogenesis with the extension of collagen fibers at both side of grafted tissue and remodeling (wound contraction and maturation) (McGavin, and Zachary, 2007). In absent of tissue rejection events, the grafted tissue (both autograft and allograft) showed a process of normal wound healing that started with hemostasis followed by acute inflammatory reaction followed by granulation tissue formation.These three steps were followed by the contraction of collagen fibers alongside bothsides of grafted tissue lead to stretching the edges and remodeling phase (Rodero and Khosrotehrani, 2010). In normal tissue histology, the skin of dogs is divided into two zones: epidermis and dermis, in which the epidermis composed from five layers, layer stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basal, while dermis composed from the superficial and deep layers of dense irregular connective tissue (Bacha and Bacha, 2012).During healing of a non-rejected tissue graft, the epidermis layer of the grafted side might be lost during the healing process, in which the epidermis can be replaced by granulation tissue (Franz et al., 2000). This replacement occurs because of degenerative and necrotic changes that occur in the epidermis layer due to hypoxia, which later overcome by the new blood vessels formation. Formation of new blood vessels is a process known as angiogenesis with the transmission of fluid from the dermis layer, albumin rich, which acts as nourishment materials until the blood circulation is restored (Bohling et al., 2006).
In conclusions, the result of the current study showed that both autograft and allograft tissue didn’t show the reaction of rejection and identically healed in a manner similar to normal wound healing.
Acknowledgments
This study was supported by the College of Veterinary Medicine, University of Mosul, Iraq. Many thanks to assistant professor Saevan S. Al-Mahmood for helping in histopathological examination.
conflict of interest
No conflict of interest.
AUthors contribution
All authors contributed equally.
References






