Advances in Animal and Veterinary Sciences
Research Article
Histopathological Study and Effect of Β-Glucan as Immunomodulater in Mice Infected by Absidia spp.
Ahmed A. Alkhalidi1, Suha Aljumaily1, Maisaa G. Taher2*, Zahid I. Mohammed1
1Department of public health ,collage of Veterinary Medicine, University of Diyala; 2Department of Pathology, College of Medicine, Diyala University, Iraq.
Abstract | This study was carried out to find the pathological changes on mice infected experimentally with Absidia spp isolated from cats, and immune modulator effects of β-glucan compound against Absidia spp. To achieve the goal, Forty mice were divided randomly into two groups, 1st group (n=20 mice) fed on diet supplement with 100mg/kg diet of β-glucan for 5weeks. 2nd group (n=20 mice) was served as a control group. The two groups were challenged with 1×107fungal cells/ml I/P of virulent Absidia spp. The result showed that 5out of 20 mice in the first group died at 1-3 days and 14 out of 20 mice in the second group. The level of protection observed in the mice of the first group showed in 75% of infected immunized mice, where 30% protection was reported in mice of the second group. The histopathological examination in the control group of mice showed acute suppurative inflammation in the examined organs at 24-72 hour post infection with necrosis, inflammatory cells infiltration and the mold and hyphae were observed in the liver, kidney, spleen and lung, as well as moderate to mild pathological lesion at day 45 post-inoculation, Mononuclear cells and neutrophils were seen in most examined organs of the 1st group. The present study was concluded that the Absidia spp. causes high pathological lesions and the β-glucan as immunomodulater alone provided partial protective immunity against lethal doses of Absidia spp.
Keywords | β-glucan, Pathological lesions, Fungal, Absidia spp, Immunomodulater
Received | January 04, 2019; Accepted | March 02, 2019; Published | May 03, 2019
*Correspondence | Maisaa G Taher, Department of Pathology, College of Medicine, Diyala University, Iraq; Email: [email protected]
Citation | Alkhalidi AA, Aljumaily S, Taher MG, Mohammed ZI (2019). Histopathological study and effect of β-glucan as immunomodulater in mice infected by absidia spp. Adv. Anim. Vet. Sci. 7(6): 508-515.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.6.508.515
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Alkhalidi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Absidia spp. is filamentous fungi that spread in nature as common contaminants in the indoor environmental. Also found in plant and soil, as well as cause food spoilage (McGinnis et al., 1999). Pathogenicity of Absidia spp. is a rare cause of human zygomycosis relatively. In immunocompetent host, Zygomycosis of Absidia spp is rarely observed. While commonly considered as an animal pathogen and may cause mycotic abortion in the cow (Ribes et al., 2000). β-glucan is found in the cell wall of fungus and bacteria as one of polysaccharide materials. The structure of β-glucanis glucose polymer linked by β-glycosidic chain (1→ 3 linear) and differs from other polysaccharides by their branching and length (Stone and Clarke,1992). β-glucans are polysaccharides found in various natural sources, such as mushrooms. due to their structure they are able to interact with innate immunity receptors, however they also act as dietary fibers in the digestive tract (Sima et al., 2018). Our immune system was stimulated by some fungi β-glucans markedly which protect us from pathogenic microbes, environmental toxins and carcinogens (Vetvicka and Yvin, 2004). Humans cannot synthesize β-Glucans, so our immune systems recognized these compounds as non-self molecules inducing both responses, innate and adaptive immune (Brown, 2011). These immunomodulatory properties primarily depend on the source they have been extracted from; studies conducted in animals and humans and confirmed that the oral administration of yeast β-glucans had a greater effect on the immune system than grain-derived β-glucans (Vetvicka and Vetvickova, 2016). β-glucan is active in all species from earthworms to humans, and is able to stimulate humoral and cellular immunity (Novak and Vetvicka, 2008) and inhibit the development of cancer (Sima et al., 2015, Barbieri et al., 2017). Prebiotic β-glucan has no harmful effect on liver and kidney functions and can be considered as immunopotentiator (immunostimulant) due to its stimulation of immune system and it has ability to reduce the adverse effect of microbial infection in rats (Somia et al., 2018). Beta-glucans from yeast and fungi have beneficial effects as supplements (Zhu et al., 2016). In contrast, β-glucans from cereals have very detrimental effects on livestock health and performance (Jacob and Pescatore, 2017).
Dietary administration of yeast β-glucans to lactating ewes caused an increase in milk yield by up to 14% (Zaleska et al., 2015). Also in ruminants, dietary supplementation with yeast β-glucans has been shown to activate macrophages (Wojcik, 2014). β-glucans interact with receptors expressed on the surface of innate immune cells and with ic3b/cR3 complement complex of opsonized cells, resulting in immune stimulation that involves innate and adaptive immunity (Sima et al., 2018). Overall, yeast β-glucans provide a valuable tool to the ruminant producer as an alternative to antibiotics (Dina and Mariët, 2018). β-glucan from rice bran could have alternative antibiotic effects and improve productivity of weaned pigs (Park et al., 2018).
The aims of present study included the histopathological changes of internal organs in mice injected with virulent Absidia spp., Also determine the influence of β-glucan as immuomodulater on viable virulent Absidia spp. in the mice.
Material and Methods
Preparation of Cultural and Biochemical Media
Sabouraud׳s Dextrose agar (SDA) and Sabouraud׳s Dextrose broth (SDB), these media was prepared according to the manufacturers’ directions.
Absidia spp Identification
This experiment was carried out in the laboratory of the College of Veterinary Medicine- University of Diyala / Iraq from the 1st of January 2018 to 1st of September 2018. Absidia spp were isolated from many infected cats.
Macroscopic Examination
Colonies morphology, color, texture, and other apparent characteristics of the colonies were examined according to (Samson and Van Reneen –Hoekstra,1988).
Microscopic Examination
The shape of mycelium, microspores, macrospores and Chlamydiaspores were determined by mixed one drop of lactophenol cotton blue stain with part of the colony on the slide then examined fewer than 40X lens (Boer and Samson, 1995).
Preservation and Activation of Isolates (3 isolates)
Reactivation of Absidia spp isolate from infected cats was done through sub cultured on Sabouraud dextrose agar and incubated at 25ºC for 4-5 days. After that, the mold growth was harvested by 5ml phosphate buffered saline pH7.2 solution and centrifuged at (2000 rpm for 10 minutes), the sediment was taken and washed for three times with PBS 7.2 and then resuspended by PBS 7.2 , by using heamocytometer chamber and red blood cells count method (Daci and Lewis,1984). The concentration of fungus suspension was adjusted to 1×107 cell/ml for Absidia spp. 0.4 ml of fungus suspension (1×107) was injected intraperitoneally into aged weak mice with poor feeding for 1-2 weeks, then killed and pure Absidia spp was reisolated from their internal organs on sabouraud dextrose agar incubated for 4-5 days, then harvested by PBS 7.2 PH and suspended, it was injected into other mice ,and this procedure was repeated for several times until a virulent isolate was obtained
Supplement of β-glucan in Mice Pellets
β-glucan in the pellets prepared by using Immunex® drug capsules which contained 10mg . The commercial pellets were grounded until becomes smooth, So 100 mg of β-glucan was added to each 1Kg of diet, to be mixed well to ensure homogenized mixture ,so it needed with distal water, then prepared as small pellets pieces. Then by using air drying, it was put in the oven air fan at 25º C for 7 hours, after that it was kept in a plastic container until use (Thomas and Van der Poel, 1996).
Experimental Design
Forty white Swiss BALB/C mice of both sexes were housed in clean cages at room temperature and divided randomly into two groups (twenty each), first group fed on a diet commercial pellet supplement with 100mg/kg of β-glucan for 5 weeks, while the second groupfed on the same diet of the first group without β-glucan and consider as a control group. After five weeks all the animals in both groups were challenged with virulent Absidia spp. then at 45th daypost challenge dose the animals were sacrificed. Gross lesions were recorded and pieces from internal organs were taken for study the histopathological changes and fungal isolates.
Preparation of Histopathological Section
Parts from different internal organs (including kidney, livers, brain, lung and spleen)were fixed in 10% formalin for several days, then the procedure of histopathological section was done at the department of Pathology stained with Hematoxyline and Eosin or PAS stain then it was examined under a light microscope (Luna,1968).
Statistical Analysis
Data were calculated with SPSS for windows TM version 24.0. as well as Chi-square and P≤0.05 were considered to be significant (Steel and Tarries,1980).
Data were calculated by SPSS for windows TM version 24.0. . as well as Chi-square, and P<0.05 was considered to be significant (Steel and Tarries,1980)
Data were calculated by SPSS for windows TM version 24.0. . as well as Chi-square, and P<0.05 was considered to be significant (Steel and Tarries,1980)
Results and discussion
Macroscopic Examination: In this test the colonies morphology characterized by the rapid growing, cottony in texture, Light Gray to creamy In color and the reverse side is uncolored or white (Figure 1).
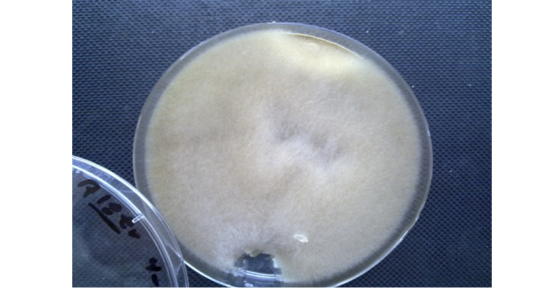
Figure 1: Absidia spp. macroscopically. Cottony in texture, Light Gray to creamy In color and the reverse side is uncolored or white.
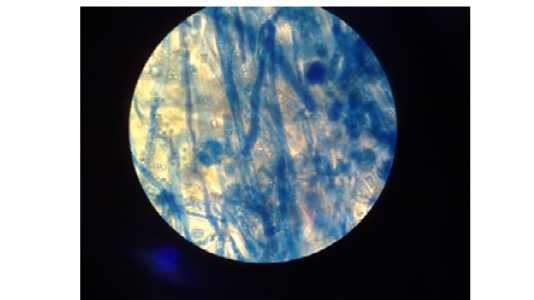
Figure 2: Absidia spp. Microscopically. Nonseptate hyphae, funnel-shaped under sporangium, sporangiophore widens and round sporangiospores.(40X lens)
Microscopic Examination: This test was used to determine the: nonseptate hyphae (distinctive point), funnel-shaped under sporangium (very distinctive point), sporangiophore widens, the sporangiophores arise on stolons from points between the rhizoids (very distinctive point) and round sporangiospores (Figure 2).
Clinical Signs and Fungal Isolation
The clinical signs in non-immunized infected animals characterized by a partial loss of appetite, depression , ruffled hair coat with death 10 out of 20mice at 48 hours post-challenge, and 4 mice died at 72 hours. The animals of this group showed heavy fungal isolation. While the 1st group mice, which fed on β-glucan only showed 25% mortality during 72 hours after a challenge dose of virulence Absidia spp (Table 1).
Heavy fungal isolation from internal organs of died mice was reported while survival mice were mild to moderate or absence of fungal isolation from internal organs was reported (Table 2).
Table 1: Explains protection percentage in mice after challenge with virulent Absidia spp.
| Groups | Number of mice | Number of dead mice | Mortality percentage | Protection percentage |
|
1st group, mice fed with β-glucan |
20 | 5 | 25% | 75% |
|
2nd group, mice control |
20 | 14 | 70% |
30% |
Table 2: Fungal growth obtained from internal organs of immunized and control infected mice.
|
Group
Organ |
1st group β-glucan |
2nd group control |
||
|
1-3 Days |
45 Days |
1-3 Days |
45 Days |
|
| Liver | ++ | + | ++++ | ++ |
| Spleen | + | + | +++ | +++ |
| Kidney | + | + | ++ | ++ |
| Brain | - | + | - | + |
| Lung | + | + | ++ |
++++ |
-- no growth.+ mild growth. ++ moderate growth. +++ heavy growth. ++++ very heavy growth.
The death of control mice during 48-72 hours after a challenge dose may indicate that animals exposed to high virulent Absidia spp that overcome the innate immune system of the host then proliferation and penetration of tissue layers at intraperitoneal cavity and they were arrived the blood stream and disseminated to internal organs mostly liver, kidney spleen and lung after that proliferation in these organs occurred and induced organs failure and death of infected animals. Zygomycetes species have great affinity for blood vessels, invade rapidly, and disseminate widely also, in all cases of severe disseminated mucormycosis the surrounding tissue the Angioinvasion with subsequent infarction is uniformly present (Kontoyiannis et al., 2005). As well as this, the pathogenic strategy of Mucorales depends on the Specific adhesion of the fungus to endothelial cells (Ibrahim et al., 2005).
The present result showed that the mice of the 1st group which fed on β-glucan express partial protection (75%), with mild-moderate growth of fungal isolates at 1-3 days post-inoculation and mild at 45 days. These data confirm the evidence of the β-glucan particles stimulate the immune system, both cellular and humeral immune enhancing properties due to activate macrophages and NK-Cells, then T-Cells, and B-Cells during selected cytokines and complement. β-glucan stimulates the immune system, and thereby have beneficial effect in fighting infections (parasitic, bacterial, fungal and viral) (Ma´rio et al., 2008). β-glucans appear to be effective at enhancing immune function and reducing susceptibility to infection and cancer (Murphy et al., 2010).
The β-glucans can be modulated both adaptive response and innate and can enhance opsonic and non-opsonic phagocytosis due to effect on several immune receptors, including Dectin-1, complement receptor (CR3) and TLR-2/6 then trigger the cellular immunity, including monocytes, neutrophils, macrophages, dendritic cells and natural killer cells (Chan et al., 2009).
Pathological Examination
Gross Pathological Lesions: Gross-examination showed no clear pathological changes in internal organs (liver, spleen, kidney, brain, lung) of control infected mice that died during 1-3 day post- inoculation, except a congestion of some organs (lung, kidney). No gross lesions were seen in the internal organs of immunized infected mice at 1-3/45 days post-infection.
Histopathological Lesions
1-Control group
Liver: The lesions in the liver of animals died during 24-48hr post infection included ,dilatation and congestion of central veins and sinusoids with neutrophils in their lumina, in addition to necrosis, thrombosis as well as neutrophils infiltration and fungal fragments surrounded by eosinophilic material in the necrotic area (Figure 3).
At 72hr post infection, the main lesions characterized by severe amyloid like substances deposition in the wall of sinusoids which led to atrophy or disappearance of hepatic cords in addition to dilating the sinusoids with neutrophils in their lumina as well as an aggregation large number of neutrophils in the capsular region with non-septet basophilic fungal hyphae were seen (Figure 4).
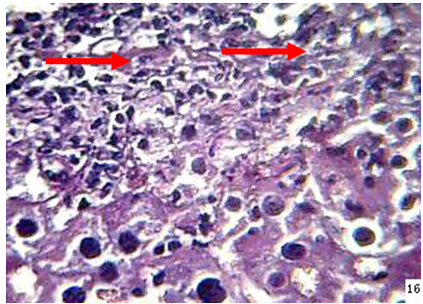
Figure 3: the liver of mouse at 48hr post infection shows, neutrophils ( ) infiltration and fungal element surrounded by eosinopohilic substances (H&E stain 40X)
) infiltration and fungal element surrounded by eosinopohilic substances (H&E stain 40X)
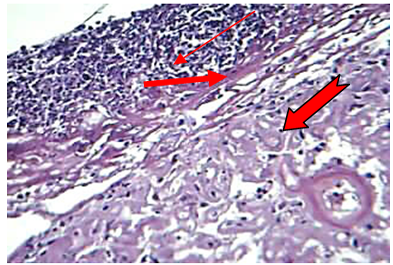
Figure 4: The liver at 72hr post infection shows amyloid (  ) like substance replacement hepatic cells with neutrophils in dilated sinusoids and central vein, fibrin (
) like substance replacement hepatic cells with neutrophils in dilated sinusoids and central vein, fibrin ( ) deposition and aggregation of neutrophils (
) deposition and aggregation of neutrophils (  ) in capsular region with fragment of hyphae (H&E stain20X)
) in capsular region with fragment of hyphae (H&E stain20X)
Spleen: The spleen expressed edema, depletion of white pulp and neutrophils infiltration were seen in animal died at 24hr post infection. Necrosis of lymphocytic cells were observed in animal died at 48hr post.
Kidney: The microscopic examination of the kidney of animal died during 24-72hr showed the vacuolation and sloughing of the epithelial lining cells of the renal tubules with hyaline cast in their lumina as well as congestion of blood vessels with neutrophils in their lumina in addition to moderate thickness of the capsular area due to fibrin deposition and neutrophils infiltration. Inflammatory cells infiltration mostly macrophages and neutrophils were recorded around blood vessels and between renal tubules of the kidney of animals died at 72hr post infection (Figure 5) As well as severe neutrophils infiltrations in capsular region with non-septated hyphae.
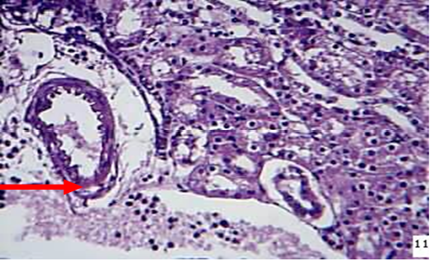
Figure 5: kidney at 72hr post infection shows neutrophils ( ) around and in the lumen of BVs between renal tubules (H&EX)
) around and in the lumen of BVs between renal tubules (H&EX)
Lung: The main lesions in the lung of animals died during 24-72hr were congestion of capillary blood vessels in the interalveolar septa with the proliferation of mesenchymal cells, in addition to congested blood vessels with neutrophils in their lumina. At 72hr post-infection, the alveolar space filled with RBCs, fibrin networks, neutrophils infiltration and non-septate irregular basophilic fungal hyphae (Figure 6).
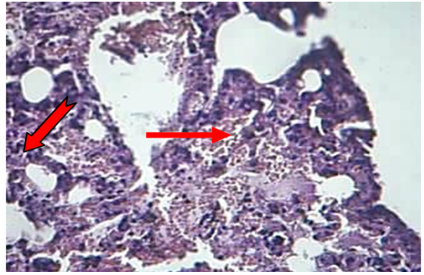
Figure 6: The lung at 72hr post infection explained fibrin (  ), RBC and neutrophils (
), RBC and neutrophils ( ) in the alveolar space and lumen of BVs (H&Estain 40X)
) in the alveolar space and lumen of BVs (H&Estain 40X)
2-Infected mice fed diet supplement with B-glucanMononuclear cells aggregation around the central veins as well as proliferation of kupffer cells are the most common lesion in the liver of the animals sacrificed at day 45th post infection (Figure 7).
Spleen: Moderate hyperplasia of lymphocytic cells in the periarteriol sheath was seen as well as moderate thickness of capsular region. Granulomatous lesion consisting from sporangium spore surrounded by macrophages lymphocyte, epitheiloid cells was seen in adipose tissue (Figure 8).
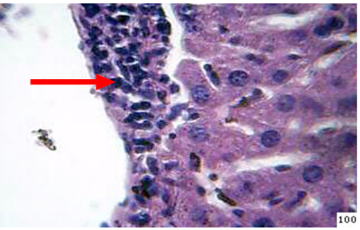
Figure 7: Histopathological section in the liver of treated animal with β-glucan at 45day post infection shows mononuclear cells ( ) aggregation around central vein and kupffer cells prolifration (H&E stain 40X)
) aggregation around central vein and kupffer cells prolifration (H&E stain 40X)
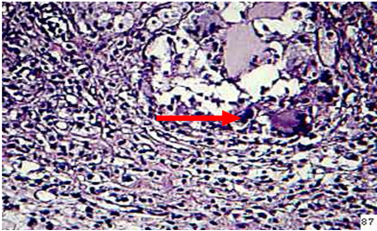
Figure 8: in the spleen of animal treated with β-glcan at 2 weeks post infection showes granulomatous lesion in adipose tissue of capsular region (H&E stain 40X)
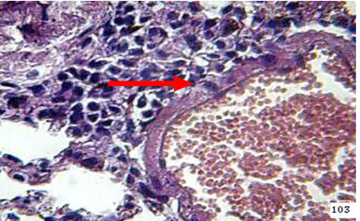
Figure 9: the lung of animal treated with β-glucan at 45 day post infection shows mononuclear cells (  ) aggregation around congestion blood vessel (H&E stain 40X)
Lung At day 45 the lung showed granulomatous lesions around blood vessels and in the interstitial tissues (Figure 9).
) aggregation around congestion blood vessel (H&E stain 40X)
Lung At day 45 the lung showed granulomatous lesions around blood vessels and in the interstitial tissues (Figure 9).
The results obtained revealed that the pathological lesions in the internal organs of infected control animals are coincident with heavy fungal isolation and 14 out of 20 animals died during 24-72 hr post-infection, this observation may indicate that the isolate of Absidia spp used in the present study is highly virulent that evades host phagocytic defense mechanism and access vasculature to disseminate to all internal organs and induced severe tissue destruction and all infected animals died.This evidence is in consistence with previous reports that explained that mucorate spp expressed a variety of virulent factors that help it evade host defense mechaniusms.
The present study showed that the most common lesions in the internal organs of the control group are supportive in nature; this result may give an indication that the mucorale antigens stimulated the phagocytic cells to release pro-inflammatory cytokines that attract neutrophils to sites of infection. However, fungal products, and lysosomal enzymes of leukocytes lead to damage the host tissues and augment suppurative reaction. this idea is in acceptance with data mentioned by Romani, (2004) and Kankkunen et al. (2010) who explained that the fungal cell wall is recognized the glucan as a pathogen-associated molecular pattern by dectin-1 and activates pro- and anti-inflammatory cytokines in a myeloid-differentiation-primary-response-gene-88 then generally leads to the nuclear factor kappa-light chain enhancer of activated B cells.
The presence of thrombosis and necrosis in most examined organs of non-immunized infected animals may indicate that the fungi invade endothelial cells of blood vessels and causes thrombosis that leads to infarction and necrosis the adjacent tissues, this observation supports the idea mentioned by Ibrahim et al. (2003) and Spellberg et al. (2005) who reported that during angioinvasion, the organism invades and damages vascular endothelial.
Fungal fragment and non-septat hyhae were seen in the lumen of blood vessels and in the center of suppurative infection, this observation may indicate that the infection disseminated via blood stream. Therefore, the infection can disseminate to other organs through the bloodstream (Park and Mehrad, 2009). Necrosis, fibrosis and granulomatous inflammation were presented in the lymph node of 20% of cattle infected with mucormycosis as well as bulbous enlargements were common in necrotic areas (Ortega, 2010).
The pathological lesions in the examined organs of infected animals supplement with B-glucan are less extensive as compared with the control group, these results may indicate that B-glucan stimulated the innate immune response which play an essential role in the stimulation of adaptive immune response and determine the outcome of the infection. The Complement Receptor 3 (CR3 or CD11b/CD18) on the surface of innate immune cells was responsible to bind with yeast and fungi, then recognizing them as “non-self (Vetvicka et al., 2007). β-glucan stimulated this receptor that leads to activate the innate immune response against pathogens (Babineau et al.,1994).
However, granulomatous reaction may indicate that B-glucan activated the cell mediated immune response that play an essential role in activating and aggregating active mature macrophages around the invasive pathogens and initiated granulomatous development, this investigation is in consistence with Yadav and Schorey, (2006) who noticed that β-glucan eliminate of infectious agents by triggers the response of immunity including proinflammatory factors production and phagocytosis. The particulate β-glucan is internalized by macrophages, which transport it to various sites throughout the body and degrading these particulate to release a bioactive soluble glucan that stimulate IL-12 production (Hong et al., 2004). According to this evidence it was suggested that the B-glucan activated the macrophages through stimulating production of IL-12 which activated NK cells to secret INF-γ that increased the activity and maturity of macrophage that elicited a granulomatous reaction. Proliferation of kupffer cells, particularly at day 45th post infection of supplement animals with β-glucan may indicate that this agent stimulated innate immune response via activated fixed macrophages this observation is in consistence with Suzuki et al. (1989) who demonstrated in an animal study administration of β-glucan to mice was found to activate the liver Kupffer cells by increasing the expression of β-glucan receptors on Kupffer cells, to produce cytokines and nitric oxide, also it anti-infective activity was tested in animal models of bacterial, parasitic fungal and viral disease (Vetvicka et al., 2007).
The presence of abscess in the kidney of animals in this group may indicate insufficient ability of defense mechanisms of the kidney to kill large number of the fungi that reach for it due to naturally decrease phagocytic cells in the kidney and the body attempts to control the infection via aggregation neutrophils which play role in eradicate of the pathogens. Also, polymorphonuclear cell abscess formation is a critical event in the response of innate immunity against invading pathogens (Mölne et al., 2000).Normal hosts can kill Mucorales by the generation of oxidative metabolites of mononuclear and polymorphonuclear phagocytes and cationic peptides defensins (Dhiwakar et al., 2003).
Moderate fungal isolation from examined organs of infected animals supplement with β-glucan may indicate that the β-glucan activated the peritoneal macrophages that killed most fungi at the site of inoculation, an administered soluble beta-glucans intravenously to mice enhanced nitric oxide synthesis of peritoneal macrophages (Hashimoto et al., 1996).
Conclusion
Absidia spp. cause high pathological lesion and showed acute suppurative inflammation in the internal organs of the mice, also β-glucan as immunomodulater alone provided partial protective immunity against lethal doses of Absidia spp.
Acknowledgements
The authors thankful all members of Department of public health - college of veterinary medicine /University of Diyala for supporting with essential requirements for present research.
Conflict of Interest
Authors declare that they have no conflict of interest.
Authors Contribution
All authors contributed equally to the manuscript.
REFERENCES






