Advances in Animal and Veterinary Sciences
Research Article
Effect of Vitamin E and Selenium Injections on the Testes and Accessory Sex Glands of Barki Rams During Non-Breeding Season
Hamed T. Elbaz*, Emad M. Abdel Razek
Theriogenology Department, Faculty of Veterinary Medicine, University of Sadat City, Menofia, Egypt.
Abstract | The aim of the present study was to determine the effect of the combination of vitamin E and selenium injection on ram reproductive efficiency. Eight mature healthy Barki Egyptian rams were injected twice/ weekly with 5 mg Sodium selenite and 450 mg vit E for one month. Ultrasound measurements of the scrotal contents and all accessory sex gland and collection of blood samples were done one week before the start of the experiment and one week after the end of experiment. Results revealed that scrotal circumference was increased significantly after treatment.The breadth of testes, epididymal tail length and breadth were increased significantly after treatment. There were significant differences in measurements of length and breadth of accessory genital glands (vesicular gland and bulbourethral gland). In addition, the breadth of ampullae and pars disseminata of prostate gland was increased significantly after treatment. Treated rams showed higher values for serum testosterone, in contrast FSH was decreased significantly. While, there was no significant difference in LH level. Our data suggest that injections combination of vit E and selenium during the non-breeding season could enhance testosterone concentrations, thereby improve reproductive efficiency of Barki rams.
Keywords | Rams, Selenium, Vitamin E, Testosterone, Ultrasonography
Received | November 04, 2018; Accepted | January 19, 2019; Published | April 08, 2019
*Correspondence | Hamed T Elbaz, Theriogenology Department, Faculty of Veterinary Medicine, University of Sadat City, Menofia, Egypt; Email: [email protected]
Citation | Elbaz HT, Abdel Razek EM (2019). Effect of vitamin E and selenium injections on the testes and accessory sex glands of barki rams during non-breeding season. Adv. Anim. Vet. Sci. 7(6): 434-440.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.6.434.440
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Elbaz and Abdel Razek. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Vitamin E and selenium are essential nutrients in sheep and cow reproduction through indispensable for many biological processes as semen quality and spermatogenesis (Jerry, 1996; Marin- Guzman et al., 1997; Yousef et al., 2003; Koyuncu and Yerlikaya, 2007). Selenium is an essential dietary trace element required for biosynthesis of testosterone to maintain male fertility (Brown and Arthur, 2001). Both testis and epididymis require exogenous supplied selenium in order to synthesize a variety of known selenoproteins which had role in spermiogenesis and post testicular sperm maturation (Ali et al., 2009). Requirements of selenium for sheep are 0.1–0.2 ppm/kg DM (NRC, 1985). In malesfed on a low selenium diet, hypogonadism was reported as well as production was reduced, semen quality was deteriorated and sperm structure and fertilization ability were impaired (Kleene, 1993; Ahsan et al., 2014). Supplementation with selenium enhanced reproductive performance in sheep (Ali et al., 2009; Marai et al., 2009). Selenium supplementation to lambs improved growth rates and testicular development in sheep (Kumar et al., 2009). Vitamin E can combat the oxidative stress by interrupting the chain reaction of lipid peroxidation and scavenging the reactive oxygen species (ROS) (Niki and Noguchi, 2004; Peris et al., 2007). Vitamin E can stabilized cell membranes containing polyunsaturated lipids (Fukuzawa et al., 1977). Vitamin E and cholesterol protected ram spermatozoa against cold shock and oxidative stress (Benhenia et al., 2016). The reproductive efficiency of growing ram lambs can be affected by nutritional status (Ghorbankhani et al., 2015). Ultrasound scanning was suitable for examination of testes and epididymis of rams, so as to interpretation of uncertain clinical findings and monitoring changes in lesions (Gouletsou et al., 2003). Ultrasound applications and post processing developments have permitted new aspects in the structural and functional analysis of testicular tissue for improvement male fertility (Schurich et al., 2009). Assessment of the reproductive ability of rams was essential part of health management in sheep flocks (Gouletsou, 2017). Accessory sex glands had very little consideration during routine breeding soundness evaluation of livestock species (Gouletsou and Fthenakis, 2010). Therefore, the aim of the present study was to determine the effect of the combination of vitamin E and selenium injection on ram reproductive efficiency during the non-breeding season of Barki rams with the help of ultrasound.
MATERIALS AND METHODS
This study was assessed and agreed by the Animal Care and Welfare Committee Ethics, Sadat City University, Egypt.
Animals
The present study was carried out on a total number of eight sexually mature rams (Barki type) belonged to the educational farm, faculty of veterinary medicine, Sadat City University, Egypt. Rams aged between 2 and 2.5 years and averaging (45-55 kg)body weight during the period from (May to June, 2018). All rams were apparently normal, dewormed and vaccinated regularly, housed in free stall barn and fed a balanced ration which formulated according to the requirement for mature ram according to (NRC, 1985) for sheep. The diet consists of 25% wheat straw and 75% concentrate mixture as well as free access of the drinking water and green fodders (Alfalfa).
Experimental Design
Animals (n=8) were injected with the combination of selenium and vitamin E (3 ml IM from Viteselen 15®, Adwia Company, Egypt), each ram injected with 5 mg Sodium selenite and 450 mg vitamin E two times / week for 1 month. Ultrasound examination and blood sampling ofall animals were done one week before conducting the experiment and kept as (control group), and then one week after end of the experiment and the same animals were kept as (treated group).
Morphometric Testicular Measurements
Scrotal circumference was measured by measuring steel tape (Ahmed and Noakes, 1995). Testis length was measured from top of the tail to the head of the epididymis for each testis using caliper (Islam and Land, 1977). All testes lengthwas done for both right and left testes.
Ultrasonographic Evaluation Of The Scrotal Contents
Ultrasonographic imaging of the testes and epididymis were done in the standing position. An ultrasound examination of rams was done by means of 5-7.5 MHz linear probe of scanner (Sonoscape-A5V, Shenzhen, China) per scrotal cutaneous to investigate the testis, epididymis and spermatic cord according to method previously described (Gouletsou et al., 2003) one week before and one week after the end of experiment. The animal restrained by an assistant to lift the tail. Scrotal hair was trimmed and shaved for evaluation of echotexture and echogenicity of testicular parenchyma. The testes should be pulled downwards within the scrotum and the examiner’s left hand is placed on the surface opposite to the one where the transducer with coupling gel is applied upon, in order to stabilize the organs. The transducer was placed on the caudal surface of the testis along its longitudinal axis (sagittal plane) and moved from right to left. The transducer was moved upwards to image the head of the epididymis and the pampiniform plexus and then the transducer moved downwards to image the tail of epididymis. The procedure was repeated for the other testis.
Ultrasonographic Evaluation of the Accessory Sex Glands
Ultrasonographic imaging of the accessory sex glands including ampulla, seminal, prostate and bulbourethral glands were scanned per rectum using ultrasound scanner with 5-7.5 MHz linear array trans-rectal probe. The transducer was fitted in a self-manufactured connector to favor its manipulation per rectum according to method previously done by (Mahmoud et al., 2013). In brief, the ultrasound transducer was lubricated with coupling gel then placed in the rectum after evacuation the feces and was moved cranially to image the urinary bladder-pelvic urethral junction as a guide. The accessory sex glands (ampulla, seminal glands) visualized near to the urinary bladder.While, pars disseminate of prostate gland visualized during the imaging of pelvic urethra. The bulbo-urethral glands appear easily at the exit of rectal probe from anal opening. The accessory sex gland dimensions were measured by ultrasound electronic caliper. Cross and longitudinal sections of pelvic urethra can be visualized. Two dimensions (dorso-ventral and cranio-caudal) of vesicular and bulbourethral glands were taken. The prostate gland can be measured by dorso-ventral dimension inside pelvic urethra. All the ultrasound machine settings including the near and far gain and contrast were kept constant throughout the study. All examinations were done by the same operator and measurements of all accessory genital glands were recorded.
Blood Sampling and Hormonal Assay
The collected blood samples (10 ml) from jugular vein were allowed to clot at 4oC for 10 h in the refrigerator then centrifuged at 3000 rpm for 15 minutes and the separated sera were stored at -20°C until subsequent analysis. Serum (testosterone, FSH, LH) concentrations were determined using ELISA kits (Calbiotech, Austin, Springer valley, CA, 91978, USA) using the micro-well method and the OD absorbance has been determined at 450 ±10 nm.
Statistical Analysis
Statistical analysis was performed using (GraphPad prism 5 software Inc., La Jolla, CA, USA). Comparison between two groups (before and after treatment) was made by independent t-test. Results are presented as means±standard errors of means (SEM). Values of p< 0.05 were considered significant.
RESULTS
Morphometric Testicular Measurements
The morphometric measures of both testes including length was increased numerically not statistically (p > 0.05). While, scrotal circumference was increased significantly after treatment (p < 0.05).
Table 1: Scrotal circumference (cm) and ultrasonographic measurements (mm) of tests, tail of epididymis and spermatic cord before and after treatment (mean ±SEM).
| Items |
Before treatment |
After treatment |
| Scrotal circumference (cm) |
28.87±1.1b |
29.81± 0.53a |
|
Testes (mm) Length right Breadth right Length left Breadth left |
51.48 ± 1.7 41.38 ± 1.7b 53.74 ± 1.5 45.47 ± 0.82b |
52.32 ± 0.92 45.80 ± 1.5a 54.22 ± 1.4 48.47 ± 1.1a |
|
Tail of epididymis Length right Breadth right Length left Breadth left |
23.77 ± 1.0b 17.23 ± 0.8b 25.26 ± 1.12b 18.68 ± 1.3b |
28.62 ± 0.6a 20.9 ± 0.9a 27.38 ± 0.74a 22.01± 0.97a |
|
Spermatic cord Breadth right Breadth left |
24.87 ± 1.90 23.13 ± 1.70 |
25.01 ± 1.99 23.61 ± 1.71 |
The values carrying different letters in the same row were statistically different (p < 0.05).
Ultrasonographic Measurements of Reproductive Organs
The ultrasonographic measurement of scrotum and its content as presented in (Table 1) revealed that breadth of both testes was increased significantly after treatment (p < 0.05) (Figure 1). The length and breadth of epididymal tail was increased significantly (p < 0.05) (Figure 2). While,there was no significant difference in measurements of the spermatic cord and length of testes after treatment (p > 0.05). The ultrasonographic measurement of accessory sex gland and hormones concentrations as presented in (Table 2) revealed that the breadth of ampullae was increased significantly after treatment (p < 0.05) (Figure 3). There was a significant difference in length and breadth measurements of vesicular glands after treatment (p < 0.05) (Figure 4). The breadth of pars disseminata of prostate gland was increased significantly (p < 0.05) (Figure 5). Also, there was a significant difference in length and breadth measurements of bulbourethral glands after treatment (p < 0.05) (Figure 6).
Table 2: Ultrasonographic measurements of accessory sex gland (mm) (ampulla, vesicular, p.disseminata of prostate and bulbourethral glands) andBlood hormone concentration (testosterone, FSH, LH) before and after treatment (mean ±SEM).
| Items |
Before treatment |
After treatment |
|
Ampulla Length right Diameter right Length left Diameter left |
46.30 ± 0.30 6.15 ± 0.28b 45.50 ± 0.40 5.84 ± 0.37b |
47.03 ± 0.4 7.26 ± 0.40a 46.21± 0.41 6.81 ± 0.34a |
|
Vesicular gland Length right Diameter right Length left Diameter left |
27.2 ± 1.40b 12.20 ± 0.37b 26.63 ± 1.67b 11.67 ± 0.50b |
29.2 ± 1.25a 13.62 ± 0.43a 29.58 ± 1.73a 12.62 ± 0.66a |
|
P.disseminata Prostate gland Diameter |
15.72 ±0.79b |
16.95 ± 0.71a |
|
Bulbourethral gland Length right Diameter right Length left Diameter left |
16.57 ± 1.0b 12.77 ± 0.49b 15.87 ± 1.03b 14.17 ± 1.05b |
18.75 ± 0.66a 14.58 ± 0.69a 17.72 ± 0.96a 15.23 ± 0.90a |
|
Hormone concentration Testosterone (ng/ml) FSH (mIU/ml) LH (mIU/ml) |
1.73 ± 0.43b 4.63 ± 0.38a 2.05 ± 0.14 |
2.88 ± 0.32a 2.93 ± 0.17b 1.80 ± 0.08 |
The values carrying different letters in the same row were statistically different (p < 0.05).
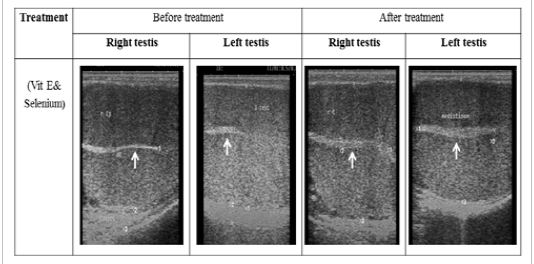
Figure 1: Ultrasonographic image of ram’s testes treated with vit E and selenium. The image shows clear increase in breadth of testes and echogenicity of mediastinum testes after vit E and selenium injection.
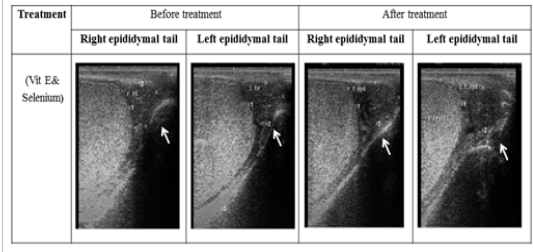
Figure 2: Ultrasonographic image of ram’s epididymal tail treated with vit E and selenium. The epididymal tail length and breadth increased after treatment (p < 0.05).
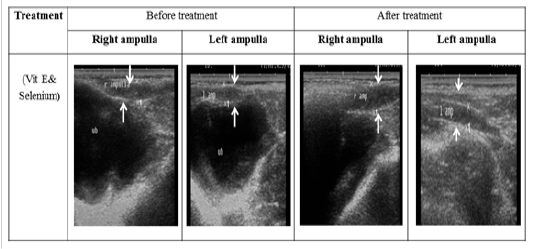
Figure 3: Ultrasonographic image of ram’s ampulla treated with vit E and selenium. The breadth of ampulla increased after vit E and selenium injection.
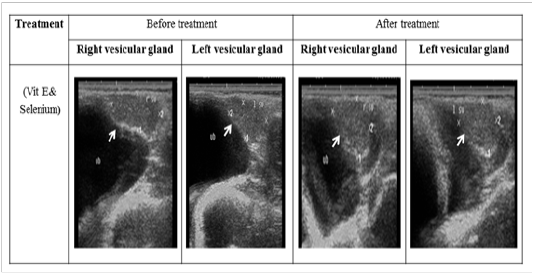
Figure 4: Ultrasonographic image of ram’s vesicular glands treated with vit E and selenium. The image shows clear increase in length and breadth of vesicular glands after vit E and selenium injection.
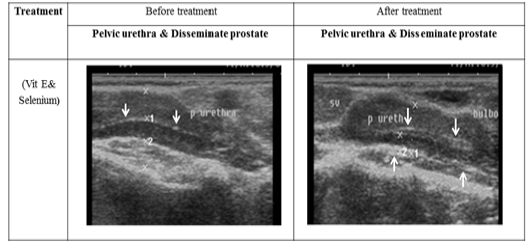
Figure 5: Ultrasonographic image of ram’s prostate gland (p. disseminata) treated with vit E and selenium.The breadth of p. disseminata enclosing the lumen of pelvic urethra increased after vit E and selenium injection.
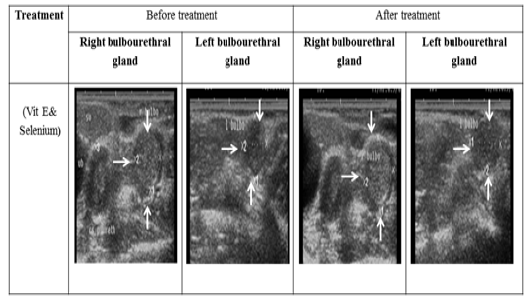
Figure 6: Ultrasonographic image of ram’s bulbourethral glands treated with vit E and selenium. The length and breadth of bulbourethral glands increased after vit E and selenium injection.
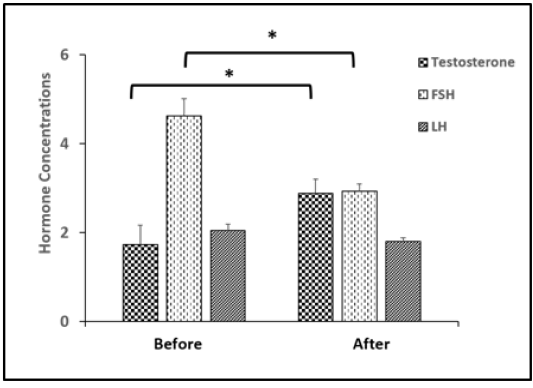
Figure 7: Changes in serum concentration of testosterone, FSH, LH in rams before and after injected with vit E and selenium.*Indicates significant difference (P < 0.05).
Hormonal Assay
Testosterone level was up-regulated significantly (p < 0.05) after treatment and FSH was decreased significantly (p < 0.05). While, there was no significant difference in LH level as presented in (Figure 7).
DISCUSSION
The reproductive efficiency of rams can be improved with vitamin E and selenium in agreement with (Ali et al., 2009; Balicka-Ramsisz et al., 2006; Kolodztiej and Jacyno, 2005; Yousef et al., 2003; Mahmoud et al., 2013). The parameters of testicular size and scrotal measurement can be used as a judgment of fertility and selection of rams before breeding (Toe et al., 2000). The scrotum circumference in the present study was measured by a tape at its widest point was (29.81±0.53) cm. In agreement with (Sargison, 2008) who reported that most mature rams should have a scrotal circumference between 30 and 40cm. Selenium improved the body weight and growth performance of sheep (Meyer et al., 2010; Neville et al., 2010). In the present study, there is a numerical increase in length of testes measurements including the right and left ones. Similar results were obtained by (Mahmoud et al., 2013). Maternal dietary selenium significantly elevated the weight, volume, length, width and circumference of testes, but not affected the testis thickness (Shi et al., 2018). The ultrasonographic measurement of scrotum and its content revealed that the breadth of testes, epididymal tail length and breadth was increased significantly after treatment. While, there was no change could be identified of the spermatic cord and length of testes after treatment. Selenium seems to be essential for testicular and Sertoli cell development in mature animals (Marin- Guzman et al., 2000; Shi et al., 2018). The testis and epididymis can synthesize a variety of known selenoproteins after exogenous supplementation which had role in spermiogenesis and post testicular sperm maturation (Ali et al., 2009). There is a close relationship between testicular size and sperm production, rams with small testes may not produce enough sperm through the mating period for maintaining good fertilizing capacity (Mahmoud, 2002). Other authors have reported that addition of inorganic selenium to the diets of rams, boars, and dairy or beef bulls did not improve their semen quality (Audit et al., 2004). This difference may be related to breed, age, condition of animal, design of treatments, nutritional status and other factors. Brzezinska-Slebodzinska et al. (1995) observed that supplementation with vit E increase the sperm cell concentration in the ejaculate. Marai et al. (2009) recorded a reduction in the reaction time in rams either dietary supplemented or injected with vitamin E and selenium. Bearden and Fuquay (1997) showed that treatment with vit E and selenium leads to an increased testosterone level that has a direct effect on secondary sexual character. Moreover, selenium is necessary for the development of germ cells in testes during development of spermatozoa and has positive effects on the number of germ cells in adults (Liu et al., 1982). Young lambs are more sensitive to selenium supplementation than lambs reaching physiological maturity (Abd El-Ghany et al., 2008). However, excessive selenium (4.0 mg/kg) can inhibit the development of testis by decreasing testicular weight and volume (Shi et al., 2018). Selenium can directly affect the leydig cell of testes, sertoli cells and indirectly affect anterior pituitary hormones secretion (Yousef et al., 1990). Vit E and selenium enhance antioxidant enzymes like (glutathione peroxidase and superoxide dismutase) and reduce the malondialdehyde (MDA) which protect Leydig cell and seminiferous epithelium from oxidative stress, lipid peroxidation and scavenging the ROS (Peris et al., 2007). In the present study there was a significant difference in length and breadth measurements of accessory genital glands (vesicular gland, prostate gland and bulbourethral gland) after treatment (p < 0.05). Similar results were obtained by (Mahmoud et al., 2013). The growth and differentiation of accessory sex glands are mainly regulated by androgens (Risbridger and Taylor, 2006). While, (Camela et al., 2017) mentioned that testosterone concentration did not vary between the peri- and post-pubertal rams and the size and function of the accessory sex glands were controlled by other factors. In the present study the treated rams showed higher values for serum testosterone after treatment. In accordance with results obtained by (Mahmoud et al., 2013). Moreover, treatment with vit E and selenium leads to an increased testosterone concentration that has a direct effect on secondary sexual character, development and maintenance of libido and mating behavior in rams (Bearden and Fuquay, 1997; Toocheck et al., 2016). Diets supplemented with selenium improve testosterone level and promote the expression of testosterone-related genes (Shi et al., 2018). Serum testosterone concentration varied with season of the year with a decrease after the autumn season and minimal concentrations during the spring. The greatest concentration was observed during the summer season (Ahmad et al., 2018). The concentration of testosterone in the current study before treatment was (1.73±0.43) ng/ml. Concentrations of plasma testosterone decreased steadily between early spring (March) to the lowest value during early summer (June) from (4.10±0.44) ng/ml to (1.82±0.10) ng/ml (Hedia et al., 2019). In the present study, FSH was decreased significantly after treatment, while, LH level was not affected and we proposed that elevated levels of testosterone might generate a negative feedback mechanism on hypothalamic-pituitary-gonadal axis. Similar results were obtained by (Matos and Thomas, 1991; Mahmoud et al., 2013) there is a close correlation between testes size with plasma LH, FSH and testosterone concentrations. Kaur and Bansal (2004) demonstrated that the levels of FSH and LH were significantly reduced during selenium deficiency. There was a difference between groups in the pattern of seasonal variation in the total LH response to GnRH (Xu et al., 1993). The seasonal changes of testicular dimensions are directed by changes of the photoperiod (Pelletier et al., 1982), which can be modified by other factors such as temperature, relative humidity and nutrition (Rosa and Bryant, 2003). The rams used in the present study were physically mature and they were effectively monitored not to fluctuate in their body weight during the study. Therefore, the changes in testes and accessory sex glands can be attributed to vit E and selenium injection. Therefore, the improvement in testes volume and upgrading of accessory sex glands dimensions could attributed to vit E and selenium injection via stimulation of total testosterone concentration.
CONCLUSION
Our results confirmed a clear positive effect of the combination of vit E and selenium injection on the scrotum circumference, testes, epididymis, accessory sex glands measurements and testosterone concentration in Barki rams. These results could be an important step to improve the reproductive performance in Barki rams by this method of supplementation during non-breeding season in Egypt.
ACKNOWLEDGEMENTS
The researchers thank the staff of educational farm, faculty of veterinary medicine, Sadat City University, Egypt for their help to conduct the current study.
CONFLICTS OF INTEREST
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
authors contribution
Both authors are involved equally in the practical part of the experiment. Hamed T.Elbaz have designed and writing of the draft manuscript and approved it in publication.
REFERENCES






