Advances in Animal and Veterinary Sciences
Research Article
Biochemical Trails Associated with Different Doses of Alpha-Monolaurin in Chicks
Nashaat G. Mustafa*
Department of Physiology, Biochemistry, and Pharmacology, College of Veterinary Medicine, University of Mosul, Iraq.
Abstract | A study was conducted with one day old 40 broiler chicks (Ross308) to investigate body performance, biochemical parameters, lipid profiles, and cytokines changes associated with the different doses of alpha-monoglyceride (monolaurin) added to the diet of these chicks in a four week period. Three doses of monolaurin (C12,FRA)® were used; 2, 4, and 8 g/kg of diet. Results demonstrated that the recommended dose (4 g/kg of diet) significantly enhances body weight gain and food conversion ratio. Otherwise, two other doses have no significant effect on the considered parameters. The novel findings of this project were the improvement of pro-inflammatory cytokines (TNF-α and IL-12) and total antioxidant capacity (TAC) by monolaurin. Also, the recommended dose has hypolipidemic influence (except on triacylglycerol). It is concluded that chicken health is critically dependent on the gut condition, nutritional status, and immune defense. The recommended dose (4 g/kg) of monolaurin enhances chick performance and immunological and biochemical manifestations.
Keywords | Biochemical parameters, Chicks, Cytokines, Lipid profile, Monolaurin.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 17, 2018; Accepted | December 03, 2018; Published | December 29, 2018
*Correspondence | Nashaat G Mustafa, Department of Physiology, Biochemistry, and Pharmacology, College of Veterinary Medicine, University of Mosul, Iraq; Email: [email protected]
Citation | Mustafa NG (2019). Biochemical trails associated with different doses of alpha-monolaurin in chicks. Adv. Anim. Vet. Sci. 7(3): 187-192.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.3.187.192
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Mustafa. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
A huge global population growth required enough dietary sources (particularly proteins) to meet the progressing demands. Consequently, poultry production advanced rapidly in many countries over the world (Scanes, 2007). On the other hand, European Union was excluded antibiotic growth promoters (AGPs) in animal foods as an attempt to overcome devastating antibiotic resistance issue (Castanon, 2007), but antibiotic residual in meat was still considered a dominant risk and challenge for both poultry producers and consumers. Accordingly, this promotes employment of various alternatives like euobiotics (Jouany and Morgavi, 2007), organic acids (Dibner and Butin, 2002), prebiotics (Gibson et al., 2017), and probiotics (Rijkers et al., 2010).
Recently, alpha-monoglycerides were consumed widely as an alternative to APGs. Alpha-monoglycerides are a family of compounds, which are formed by a fatty acid attached to the first, usually called alpha, carbon of the triacylglycerol via a covalent bond by esterification (Bedford and Gong, 2018). Although many kinds of fatty acids can be contributed to the alpha-monoglycerides like; propionic (C3H6O2), butyric (C4H8O2), caprylic (C8H16O2), and capric (C10H20O2), but lauric acid (C12H24O2) is the favorite one, making alpha-monolaurin (in short called monolaurin). Interestingly, lauric acid occurs in the nature abundantly forming about 50% of the coconut oil (Liau et al., 2011), also monolaurin has characters of both fatty acids (lipid soluble) and glycerol (water soluble), that means amphiphilic compound, hence it can be administered orally with drinking water or diet of poultry (Carpo et al., 2007).
Furthermore, antiprotozoal, antifungal, antibacterial (Harrison et al., 2013) and antiviral (of Marek’s disease, Newcastle disease, infectious bursitis, avian influenza, and others) (Thormar et al., 1987; Boddie and Nickerson, 1992; Haase et al., 2015) activities of monolaurin reflected the unique features that put it on the top of the food additives. Also, the inability of bacteria to develop resistance against monolaurin was considered vital in attracting more attention to this compound (Ruzin and Novick, 2000). Indeed, it was recorded that monolaurin is non-corrosive, non-volatile, suitable taste and flavor, heat-stable (up to 160 °C), pH stable and not dissociated in the intestine (Harrison et al., 2013).
It is hypothesized that different doses of monolaurin added to the food of chicks could have various effects on the body performance, and biochemical parameters. Although, the progressive utilization of the monolaurin as a defender against the pathogenic microorganisms, and body performance enhancer, unfortunately, according to the knowledge of the author, there was no work in chicks to investigate the biochemical profile influenced by this substance. Hence the aim of this project was to explore body performance, biochemical parameters, lipid profiles, and cytokines alterations related to the different doses of monolaurin added to the diet of these chicks. Additionally, because of the expanded utilization of the monolaurin with fascinating consequences, many poultry producers may not adhere to the use of a recommended dose, and this needs more elucidation. In this article, it is recorded that the recommended dose of monolaurin enhances chick body performance, biochemical parameters, and immunological markers, in contrast to half and double doses, which have no obvious effects. The novelty of these results was a promotion of the pro-inflammatory cytokines (TNF-α and IL-12), and total antioxidant capacity (TAC) and decrease levels of serum cholesterol and LDL by a monolaurin.
Materials and Methods
This project was carried out on March-August 2018 in the animal house unit and laboratories of the college of veterinary medicine, University of Mosul. One day old forty healthy chicks (Ross 308) were achieved from a local hatchery and allocated to the floor, no vaccines, drugs, or chemical treatments were used. Chicks housed under standard suitable conditions with continuous lighting, they provided starter diet from day 1 to 20, and grower diet from day 21 to 28 (NRC, 1994) (Table 1). Birds allotted randomly into four groups (n=10 for each group); control group with no treatment, and three groups treated with monolaurin (C12, FRA®, Netherlands) for four weeks with the recommended (by manufacture FRA®) dose (4 g/kg), half dose (2 g/kg), and double dose (8 g/kg) of food, water and diet ad libitum. All experimental works were performed according to the care of animals as guided locally by the college of veterinary medicine, University of Mosul, and all established and international guidelines for the use and care of laboratory animals were committed. Body weight gain (g) and average food intake (g) were calculated weekly, and depending upon them, food conversion ratio (FCR) = body weight gain (g)/food intake (g), were determined.
Table 1: Composition of the experimental diet
| Ingredients | Starter diet (%) days 1-20 | Grower diet (%) days 21-28 |
| Corn | 61.2 | 71.2 |
| Soybean meal | 35 | 25 |
| Dicalcium phosphate | 1.9 | 1.9 |
|
NaCl |
0.4 | 0.4 |
| Limestone | 1 | 1 |
|
Vitamin mixa |
0.25 | 0.25 |
|
Mineral mixb |
0.25 | 0.25 |
| Total | 100 |
100 |
aVitamin mix was composed of (amount in 1 g): vitamin A: 400 IU; vitamin E: 5 IU; Biotin: 0.02 mg; Cyanocobalamin: 1 μg; cholecalciferol: 100 IU; Menadione sodium bisulfite: 0.05 mg; Folic acid: 0.2 mg; Nicotinic acid: 3 mg; Calcium pantothenate: 1.6 mg; Thiamin-HCl; 0.6 mg; Pyridoxine-HCl: 0.7 mg; Riboflavin: 0.6 mg; bMineral mix was composed of (amount in 1 g): Na: 0.1 g; Mg: 0.05 g; S: 0.03 g; Cl: 0.16 g; I: 0.02 mg; Cu: 0.6 mg; Fe: 4.5 mg; Se: 0.02 mg; Mn: 5.9 mg; and Zn: 2.9 mg.
At the end of the experiments (week 4), blood was collected from the jugular vein, and serum separated by centrifugation 3000xg for 15 minutes (Thermo Scientific CL10 Centrifuge, USA). Serum biochemical analysis were done using commercial kits enzymatic methods (722N UV-VIS spectrophotometer, China) to determined serum glucose, total protein, uric acid, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, total antioxidant capacity (TAC), and lipid profiles (total cholesterol, triacylglycerol, low density lipoproteins (LDL), very low density lipoproteins (VLDL), high density lipoproteins (HDL) (Burtis et al., 2006). Also, Elisa kits specific for chickens were used (3550-UV microplate reader Bio-Rad, USA) to estimate chicken tumor necrosis factor-alpha (TNF-α) Elisa kit (Cat No. MBS7606967), chicken interleukin-12 (IL-12) Elisa kit (CAT No. MBS2501716) (Burtis et al., 2006).
Statistical analysis was achieved by SPSS program and one way analysis (ANOVA) test was used to compare between means (mean ± standard error) and Duncan’s test to evaluate the significant differences between means, were considered at (p≤0.05 and p≤0.001) (Bass, 2007).
Results
In this study, it is sought to investigate the effects of three doses of monolaurin added to the diet of chicks for 28 days on body performance, and certain biochemical and immunological parameters. It is evident from the results that there were considerable variations in the measured param-
Table 2: Body performance of four groups (mean ± standard error of n=10 chicks.).
| Groups |
1st week |
2nd week |
3rd week |
4th week |
|
| Body weight gain (g) | Control |
108±7.6c |
222±8.6b |
306±13.7c |
425±17.0c |
| 2 g/kg |
129±6.5b |
224±9.7b |
319±12.1b |
428±18.4c |
|
| 4 g/kg |
143±7.2a |
233±9.4a |
341±11.9a |
481±21.3a** |
|
| 8 g/kg |
134±6.1b |
227±7.4b |
315±12.4b |
430±17.1c |
|
| Average food intake (g) | Control |
225±9.3c |
448±12.2a |
633±18.8b |
862±27.5a |
| 2 g/kg |
268±10.0a |
454±13.3a |
667±19.5a |
881±28.3a |
|
| 4 g/kg |
260±9.9a |
420±14.6b |
623±16.7b |
875±33.6a |
|
| 8 g/kg |
270±10.3a |
461±12.9a |
634±16.7b |
869±28.2a |
|
| Food conversion ratio (FCR) | Control |
2.08±0.02a |
2.01±0.01a |
2.06±0.02a |
2.02±0.05a |
| 2 g/kg |
2.07±0.06a |
2.02±0.02a |
2.09±0.05a |
2.05±0.04a |
|
| 4 g/kg |
1.81±0.02c** |
1.80±0.04c** |
1.82±0.02c** |
1.81±0.03c** |
|
| 8 g/kg |
2.01±0.03a |
2.03±0.06a |
2.01±0.04a |
2.02±0.03a |
Different letters means a significant differences (p≤0.05) between groups of the same age (columns).
**highly significant differences (p≤0.001) between groups of the same age (columns).
eters. Noticeably, the recommended dose 4 g/kg diet has augmenting effects on the body weight gain, food conversion ratio, and fluctuated effects on the serum biochemical and lipid profiles, with the improvement of IL-12, and TNF-α. Body weight gain of 4 g/kg group chicks was increased significantly (p≤0.05) since week 2, and up to highly significant (p≤0.001) to week 4, in contrast, other two groups have insignificant changes when compared with control (Table 2). Meanwhile, there was a highly significant elevation in food consumption at week 2 and 4 of the group of 4 g/kg chicks, while unnoticeable changes recorded in other groups. FCR also improved (p≤0.001) in the group of 4 g/kg during experimental periods (Table 2).
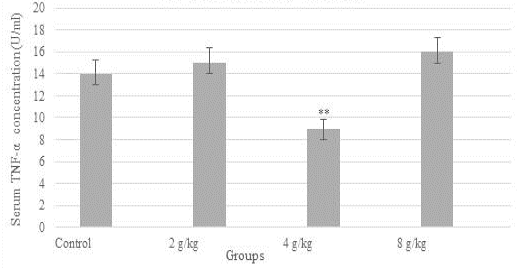
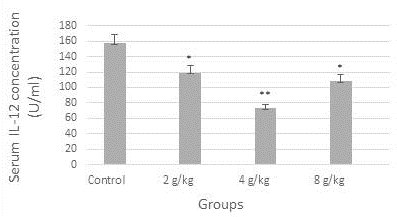
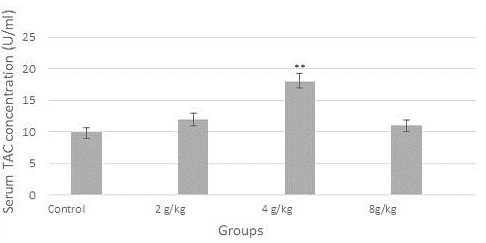
Figure 1 demonstrated a reduction in the concentration of serum TNF-alpha (9 U/ml) significantly in the group of the recommended dose (4 g/kg), likewise, the concentration of IL-12 was diminished significantly (p≤0.05) in all dose groups when compared to control (Figure 2). In contrast, as shown in Figure 3, serum TAC concentration was increased markedly in the group of 4 g/kg.
On the other hand, it was apparent that all doses of monolaurin have inconsiderable effects on serum glucose, uric acid,and creatinine. In contrast, serum total protein, AST, and bilirubin were increased (p≤0.05), and there was an abundant elevation in the ALT and bilirubin at the group of 8 g/kg (Table 3). However, serum lipid profile of chicks generally fluctuated according to the dose of monolaurin, as indicated in Table 4, there was a marked elevation in the level of triacylglycerol, and VLDL, with appreciable depression of the cholesterol, LDL and HDL levels.
Table 3: Serum biochemical parameters of four groups (mean ± standard error of n=10 chicks).
| Control | 2 g/kg | 4 g/kg | 8 g/kg | |
| Glucose (mg/dl) |
310±9.4b |
298±8.9b |
302±9.3b |
307±10.1b |
| Total protein (g/dl) |
2.9±0.4b |
2.7±0.3b |
3.7±0.6a |
3.9±0.4a |
| Uric acid (mg/dl) |
6.2±0.09a |
6.4±0.12a |
8.5±0.08a |
8.8±0.14a |
| Creatinine (mg/dl) |
0.9±0.02b |
0.9±0.02b |
1.3±0.02a |
1.4±0.02a |
| ALT (U/L) |
14±1.3c |
13±1.4c |
17±1.3b |
23±1.6a** |
| AST (U/L) |
116±6.8b |
109±6.0b |
121±7.3b |
137±6.1a |
| Total bilirubin(mg/dl) |
7.8±1.3c |
8.1±2.0c |
9.2±1.9b |
12.8±0.27a** |
Different letters means a significant differences (p≤0.05) between groups (rows) compared to the control.
**highly significant differences (p≤0.001) between dose groups (rows) compared to the control.
Table 4: Serum lipid profile of four groups (mean ± standard error of n=10 chicks).
| Control | 2 g/kg | 4 g/kg | 8 g/kg | |
| Triacylglycerol (mg/dl) |
87±3.2c |
90±2.9c |
113±3.7b |
137±5.0a** |
| Cholesterol (mg/dl) |
109±4.1a |
113±5.2a |
91±4.3b |
116±5.7a |
| LDL (mg/dl) |
55±2.5a |
59±2.1a |
43±3.3b |
60±2.8a |
| VLDL (mg/dl) |
36±1.1c |
38±1.7c |
45±1.2b |
58±2.0a** |
| HDL (mg/dl) |
70±2.1a |
72±1.9a |
71±2.9a |
54±2.3b |
Different letters means a significant differences (p≤0.05) between groups (rows) compared to the control.
**highly significant differences (p≤0.001) between dose groups (rows) compared to the control.
Discussion
This study provides an evoked evidence that monolaurin was one of the encouraging food additives in poultry production to improve broiler performance at the recommended dose (4 g/kg), also there was perfection in the pro-inflammatory cytokines (TNF-α and IL-12) and certain serum biochemical parameters, but unfortunately the recommended dose causes elevation, but has non-deterioration effects, in the serum triacylglycerol and VLDL. By contrast, half (2 g/kg) and double (8 g/kg) doses of monolaurin have no preferring significant effects on the studied parameters.Improvement in body weight gain and FCR of the recommended dose of monolaurin may be due to an enhancement of alimentary tract digestive enzymes activity, also the process of digestion, and absorption (Yegani and Korver, 2008). Moreover, encouragement of gut health and resistance to the gut disorders by monolaurin result in the amelioration of body performance, and these outcomes agree with Zeitz et al. (2015). Elevation of body weight gain and FCR without an increase in food intake (Table 2) may be due to the improvement of gut efficiency by monolaurin. Authors suggested that monolaurin (alone or with metronidazole) participate in the healing process of intestinal epithelia after experimental Giardiasis infections (Fahmy et al., 2014), this may explain the gut enriching impact of the monolaurin.
Enhancement of the pro-inflammatory cytokines (TNF-α and IL-12) and total antioxidant capacity (TAC) (Figure 1, 2 and 3) is one of the novel findings of this paper, and it may be due to an enhancement of general body condition and nutritional status accompanying with boosting of the chick immune system. TNF-α is the main cytokine that plays a chief role in the animal defense mechanism particularly during inflammatory processes (Calder, 2002; Rohde et al., 2018). These results were in good agreement with Fahmy et al. (2014) who has recorded the improvement of pro-inflammatory cytokine concentrations like TNF-α, IL-10, and IL-4 by the monolaurin in hamsters. It is believed that monolaurin modulate the inflammatory cytokines expression, proposing it may have a modulatory impact on the host pro-inflammatory response to support eliminate the bacterial infection (Silva et al., 2018). While, Seleem et al. (2016) documented that certain cytokines not affected by monolaurin (as IL-8), whereas others were declined, and this may be due to body response heterogeneity to monolaurin via diversification of the gene expression regulation of the pro-inflammatory cytokine genes (Seleem et al., 2016). On the other hand, many studies have suggested that oxidative stress in birds may be involved in diseases affecting animal production. For example, the humid and hot situation in birdcages may cause heat-induced oxidative stress in birds, which in turn shrinks growth and meat quality (Moradi et al., 2016). Thus, the antioxidant activity of food additives plays a key role in poultry production. TAC level elevation may be due to enhancement effects of monolaurin on gut activity, pro-inflammatory cytokines, antimicrobial and antiviral properties.
For obscure reasons, chickens have a higher serum glucose level than other vertebrates of the identical body mass. Glucose is absorbed by chick gut through glucose transporter proteins (GLUTs) and Na-glucose transporter (Braun and Sweazea, 2008).While creatinine is the metabolic product of muscle metabolism. It can be observed in Table (3), the changes in the serum levels of glucose and creatinine were negligible, and this finding may indicate that monolaurin has no influence on the metabolism of glucose and creatinine. Compared to other animals, birds are considered hyperglycemic and typically have blood glucose level that is twice that of healthy humans (Bai et al., 2017), “chicken normal hyperglycemia” may explain a relative steady state of blood glucose in treated groups. Otherwise, elevated serum total protein and uric acid may indicate encouraged metabolic rate and subsequent products, hence it is believed that serum uric acid is indicated of nitrogen (particularly amino acids) metabolism (Donsbough et al., 2010).
Raised levels of ALT, AST, and bilirubin in the high dose (8 g/kg) group (Table 3) could be due to harmful effects of a high dose of monolaurin on the hepatic tissue, these results disagree with Seleem et al. (2016) who documented that monolaurin was safe during their experiments, and it did not show any toxic activity in higher concentration (up to 2500 µM) on the tissue culture cells (fibroblasts).
As detailed in the Table (3), it seems that the fluctuations in the lipid profile parameters may be dose-dependent.Triacylglycerol as a well-known storage form of an excess amount of lipids and carbohydrates, seem to be raised in the serum of treated groups and this outcome may be due to the direct or indirect effects of monolaurin on the triacylglycerol metabolism, leading to the elevated concentration of serum triacylglycerol. Although the main serum VLDL is of hepatic origin, VLDL is rapidly catabolized by lipoprotein lipase (Rodwell et al., 2015). However, the results agree with Eyres et al. (2016) who has suggested that monolaurin influence on the lipid profiles could be due to its action on the hepatic lipid metabolism. Monolaurin is recognized to act as a hypolipidemic and a hypocholesterolemic, in addition, it has antidiabetic and antioxidant properties.Otherwise, it’s also reported that monolaurin was elevated triacylglycerol, VLDL, and HDL and depressed LDL (Resende et al., 2016). From this study, we concluded that monolaurin in the recommended dose (4 g/kg) improves immunological and biochemical markers and chick performance. These findings are promising but future work should focus on certain gaps in our project, for example, a detailed effects of recommended and a toxic dose of monolaurin, particularly on the intestinal absorption, liver metabolic pathways, and more immunological biomarkers.
AcknowledgementS
The author would like to thanks the College of Veterinary Medicine, University of Mosul, Iraq, for it is supported in applying this paper.
Conflict of interests
The author declares that there is no conflict of interest.
References