Advances in Animal and Veterinary Sciences
Research Article
The Influence of Iraqi Propolis on the Cutaneous Wounds Healing in Local Breed Dogs
Hussein Ibraheim, Dhurgham Hayder*
Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Kufa, 54003, Najaf, Iraq.
Abstract | Propolis “bee glue” is a nature substance, it is collected from combing of bee hive frames. It has several medical remedies such as anti-microbial, anti-spasm, local anesthetic, and wound healing. The cutaneous wounds repair still remain a big challenge because of skin ability to heal as scar. Therefore, this study aimed to evaluate the Iraqi Propolis to regenerate wound healing. To estimate the total leukocyte count (TLC), wound diameter, histopathological examination of the skin and evaluation of cutaneous fibroblast growth factor (FGF2) level. Results revealed that the TLC recorded significant elevating in control group for all periods after induced wound. While the treated group was returned to normal level in (21 and 28 days). The wound diameter was reduced in the treated group compered with the control group. The histopathological changes of treated group rebounded to a semi-natural pattern in analogical periods compared with control group. The FGF2 level suffered from rapid elevating with small period in propolis group more than control group to heal the wound. Also its increase rebounded the healed cutaneous wounds at last periods of the treated group to regeneration. This assay demonstrated propolis is not only enhancement and acceleration of the wound healing in dogs but also prevent contamination, relive pain, avoid scar formation and minimize hemorrhage therefor it is a typical drug for a topical administration of the wound treatment.
Keywords | Bee glue, FGF2, Growth factor, Regeneration, Skin repair, Skin structure
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | August 07, 2018; Accepted | September 19, 2018; Published | October 04, 2018
*Correspondence | Dhurgham Hayder, Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Kufa, 54003, Najaf, Iraq; Email: [email protected]
Citation | Ibraheim H, Hayder D (2018). The influence of iraqi propolis on the cutaneous wounds healing in local breed dogs. Adv. Anim. Vet. Sci. 6(11): 514-520.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.11.514.520
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Ibraheim and Hayder. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Skin is the major organ covering the whole body which performs many functions such as defense, temperature balance, sensation, secretion, synthesis of vitamin D, storage of fat or lipids, and pigmentation (Shumaker, 2015). It consists of three layers: Epidermis which contains the stratum basale. It is composed by a germinative keratinocytes in a single row as well as forms melanocytes. Dermis is the second layer of skin which composes of reticular matrix connection (collagen and elastic fibers), cells (histiocyte, fibroblast and mast cell), arrectores pilorum, vessels and appendage structures (nails, hair and sebaceous gland these extend to epidermis) (Figure 1 & 2). Hypodermis is a deepest layer of skin is consisted by a connective tissue, elastic fibers and adipocytes (Serra et al., 2007).
Wound healing is a physiological dynamic and biological complex processes to repair of soft tissues including soluble mediators (growth factors and cytokines), blood cells (leucocytes, thrombocytes and endothelial cells), extracellular matrix (collagen, glycosaminoglycans, elastin, proteoglycans and hyaluronic acid) and parenchymal cells (fibroblasts, angiocytes and keratinocytes). It has three classic phases: inflammatory, proliferative and maturation phase (Singer & Clark, 1999). The inflammatory phase is started immediate after injury until 2-4 days. This phase includs two responses: the vascular and inflammatory response. The vascular response describes by: 1) The damaged vessels. 2) Secretion of platelet vasoconstrictor mediators. 3) Formation of hemostatic plug due to platelet aggregation by activated of adhesive glycoproteins with fibrin net and finally. 4) Releasing growth factors and cytokines to initiating the next response. While the inflammatory response recognizes by a redness, swelling, heat, pain and lost function because of leaking the plas ma and neutrophils (first
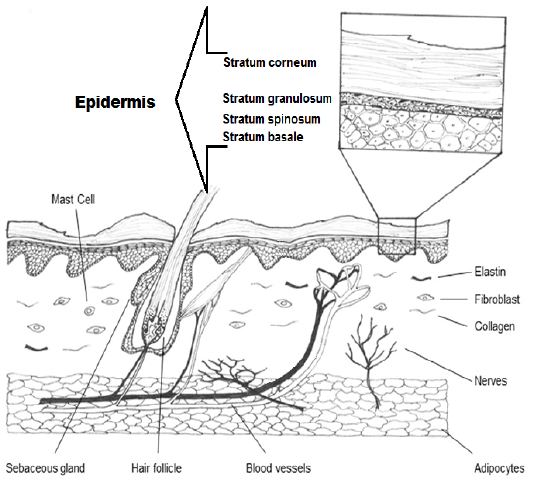
Figure 1: Diagram shows the epidermis with stratum corneum, stratum granulosum, stratum spinosum and stratum basale. Also dermis with its structures in the cutaneous dog.
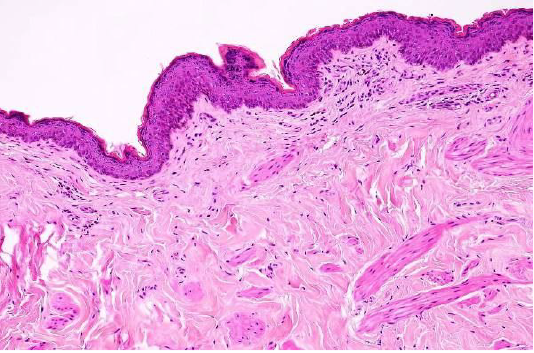
Figure 2: Showed the normal structure of epidermis and dermis in the cutaneous dog (H&E×40) (Dirrig et al. 2016).
defense line) into the tissue that activate as cleaner of debris and infection this stimulates mast cells which distraction of fibrin that lead to recall the macrophages (second defense line). The macrophages secrete a different chemotactic and growth factors (transforming growth factor “TGF”, epidermal growth factor “EGF”, FGF, and interleukin-1). The proliferative phase is begun from 4-21 days which included three responses: the granulated, contracted and re-epithelized response. The growth factors and cytokines stimulate the fibroblasts to migrated and proliferated from the surrounding tissues of injury which exploits the wound extracellular matrix to building granulation tissue (Flanagan, 2000). Furthermore, the fibroblasts product collagen fibers and that altered into myofibroblasts which have highly actin microfilaments to contracted of wound edges (Werner & Grose, 2003). The epithelial cells divide and migrate from the wound edges into hole to create granulation tissue. While the hair follicles, remnants and sebaceous glands that surrounding the granulation or scar tissue (Flanagan, 2000). The growth factors are the proteins synthesis and store at the granules in the various cells such as platelets, macrophages, lymphocytes, keratinocytes endothelial and mast cells which play great roles in the healing processes. These growth factors involve FGF is stimulated keratinocyte, fibroblasts proliferation and migration, and also angiogenesis, EGF is stimulated keratinocyte and fibroblasts migration, keratinocyte growth factor “KGF” is stimulated keratinocyte migration, differentiation, and proliferation and VEGF is stimulated angiogenesis (Broughton et al., 2006). The maturation remodeling phase occurs within 21 days to 2 years by gradually degradation of the collagen type III and replaced into collage type I which converts scar to normal tissue (Kumar et al., 2013).
Propolis is a Greek term means the city defender, it looks like a gum and also known as bee glue. Propolis is collected from different plants by bees which varies in color from greenish yellow to dusky brown which may lead to the comb staining/frame and perhaps found at extracted honey (Burdock, 1998). Propolis acts as many biological activity like antibacterial, antifungal, anti-tumor (antimetastatic or antiproliferative), antiviral, antiparasitic, antioxidant, antitoxic, imunostimulant, anti-inflammatory activity (Farre et al., 2004). It consists from resin, balsam, wax, etheric oil and pollen. Also Propolis contains minerals (calcium, manganese, copper and zinc), amino acids, vitamins (C, E and B group), and flavonoids (Cardinault et al., 2012; Abbass et al., 2009). The animals are exposure for many events harmful daily especially dogs because of its excitement nature, direct attrition with environment, guarding and hunting. Therefore, this study aimed to evaluate of Iraqi propolis capability for wound healing activity.
Materials and Methods
Experimental Animals and Biochemical Reagents
Sixteen healthy local breed of the male dogs 6-8 months in age were used for this experiment. Xylazine 2% (Hoge Mauw/Belgium), Ketamine 10% (Fabrique par: KEPRO P.V./Holland), Iraqi Propolis, Vaseline (Syria), Ethanol 70% (Scharlab S.L./Spain), TRIzol Reagent (Thermo Scientific/USA), Filter paper (Ahlstrom/USA), Hemocytometer slid chamber (Neubauer Improved/ German), Mic qPCR Cycler (Bio Molecular System/Australia) and Microscope (Olympus/ Philippines).
Experimental Design
The dogs were divided into two groups and each group comprising 8 dogs. They were housed in (1×1.25) meters cages individually. They placed in the animals house of the Veterinary Medicine Faculty of Kufa University. The animals feeded by the balance food and managed from the professional team. The first (treated) group and second (control) group subscribed for induced skin open wound as a follow:
Open Wound Model
The right costoabdominal area was prepared for aseptic wound creation. The area was marked four circle shape 2cm in diameter. Xylazine 5mg/kg BW and ketamine 15mg/kg BW intramuscularly were injected to anesthetized dog. Whole thickness of the circle skin areas was incised and removed. The bleeding was haemostasis via clamp forceps and digital gauze pressure. The lesion areas were tied with sterile gauze and Cryp-bandage. That procedure applied for both groups while in the treated group placed Propolis extracted ointment before tying.
Propolis Extracted Ointment Preparation
The raw propolis collected from different aperies of Najaf city at Iraq. That propolis was macerated with ethanol alcohol 70% to extracted pure propolis. This mixture filtrated by filter paper and concentrated by water bath at 50oC (Margeretha et al., 2012). Propolis extracted mixed with equal weight of a vaseline.
Parameters
The wound healing in two groups were evaluated by measurement of the Total Leukocyte Count (TLC) before (0 period) and after induced wound at (7, 14, 21, and 28 days). The healing degree assessment grossly measured the wound diameter at (1, 7, 14, 21 and 28 days). The histopathological exam was performed to differentiate between these groups by taken 1cm3 of the healing areas after 28 days. Furthermore, the FGF2 was estimated from the peripheral egad wounds of the skin samples at (0) period means pre-operation and (7, 14, 21 and 28 days) post-operation. The 0.25cm3 samples of the skin were placed at TRIzol reagent, labeled and frozen at -20ºC until measured. The growth factor concentration with analysis and calculation of gene expression levels depend on RNA concentration after conversion it to copies DNA, all processes including total RNA purification, qPCR amplification and data analysis. The FGF2 percentage estimated utilizing standard curve (Figure 3).
Statistical Analysis
It was performed by using statistics software (SPSS) 18.0.0, with the one way analysis of variance (ANOVA). Least significant difference (LSD) was applied to compare between means. The value p < 0.01 is consider to be significant.
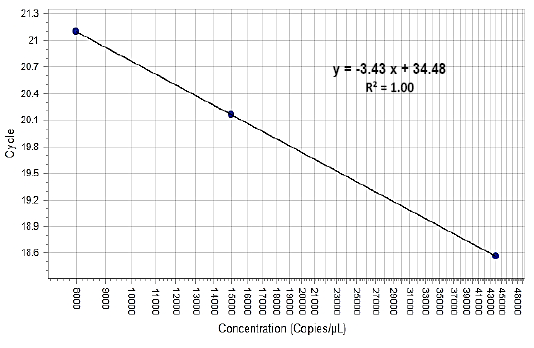
Figure 3: Showed the standard curve
Results
The TLC increases significant difference at p<0.01 level in both groups compared with (0) period that elevating in the control group comprised (7, 14 and 21 day) while returned to same significant level at 28 day. However, the treated group rebounded at periods of (14, 21 and 28 day) and that elevate occurred only in the 7 day (Table 1).
Table 1: Total leukocytes count/ml.
| Period /day | Control group (M±SE) | Treated group (M±SE) |
| 0 | 6487.5±68.8 A | 6362.5±77.4 A |
| 7 | 11687.5±65.7 B | 10425±176.2 B |
| 14 | 9550±218.9 B | 7562.5±163.8 A |
| 21 | 8662.5±139 B | 6637.5±132.9 A |
| 28 |
7112.5±124.8 A |
6400±130.7 A |
The large letters denote significant difference at p<0.01 level.
The healing degree assessment was observed by wound diameter measurement which was showing gradually declined with a significant difference at (p < 0.01). It was initiated from 14 to 28 days for the treated group with significant difference among them but the control group appeared the significant level decreasing only at periods of the 21 and 28 days without showing significant difference between them (Table 2).
Table 2: Wound diameter measurement/cm.
| Period /day | Control group (M±SE) | Treated group (M±SE) |
|
1 |
2±0.06 A | 2±0.05 A |
| 7 |
2.19±0.04 A |
1.98±0.04 A |
| 14 | 1.98±0.04 A | 1.59±0.07 B |
| 21 | 1.68±0.08 B | 1.18±0.04 C |
|
28 |
1.39±0.09 B |
0.69±0.07 D |
The large letters denote significant difference at p<0.01 level.
The data of the present study revealed that the edge of wound lesion was reduced and it looked like volcano orifice protrusion at day seven in the treated group. While the reduction of the edges were at day 14 in the control group (Figure 4). Furthermore, some cases of the control group were suffering from suppurative exudate formation as a result of contaminated induce wound lesion with pyogenic microorganisms (Figure 5).
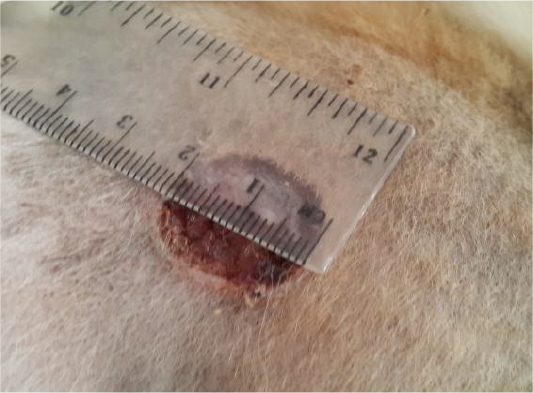
Figure 4: Showed like volcano orifice protrusion
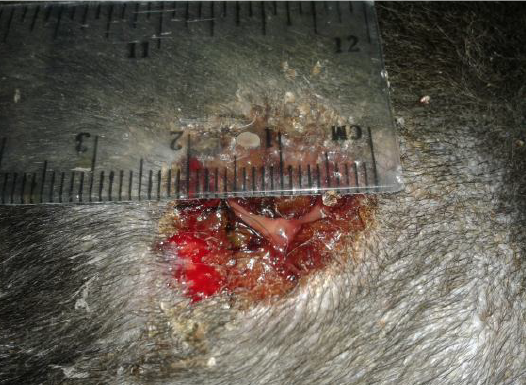
Figure 5: Showed the suppurative exudate formation
In the histopathological exam the epidermis of treated group was observed the typical stratum basale layer with arranged the stratum spinosum and granulosum as attempt to rebound to a normal pattern and it also covered with a thin keratinizing stratum corneum layer. The dermis was consisted from great thick granulation tissue which involved the proliferative and migrated cells “fibroblasts, mast and macrophages” with a few number of small vessels inside the fibrous reticular matrix that arranged parallel to the tissue surface with absented appendage structures (Figure 6). Some samples proved convert from irregular arrangement to regular pattern (Figure 7) when compared with the normal structure in (Figure 1 & 2).
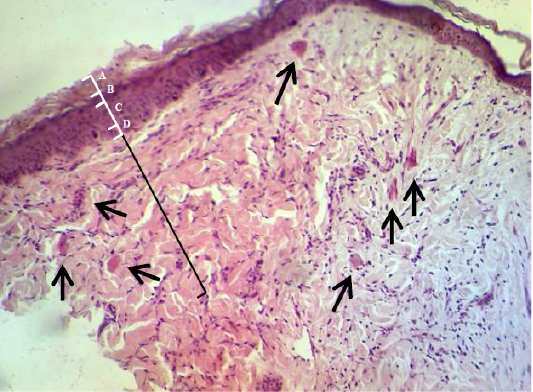
Figure 6: Showed the epidermis (white ruler) stratum corneum (A), stratum granulosum (B), stratum spinosum (C) and stratum basale (D). Dermis (black ruler) blood vessel (black arrow). The preparation was by (H&E×40).
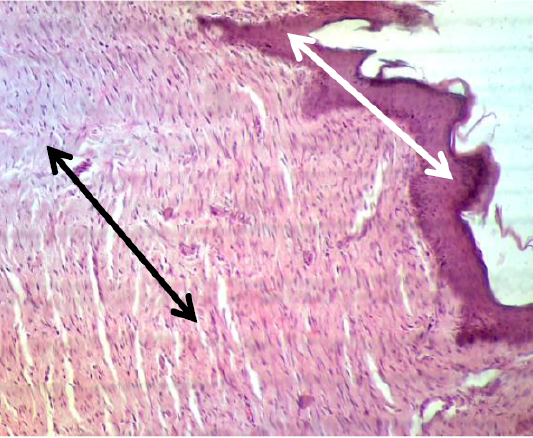
Figure 7: Showed the epidermis (white arrow) and dermis (black arrow) convert from irregular arrangement to regular pattern. The preparation was by (H&E×40).
However, the histopathological changes of the epidermis in control group were showed interrupted stratum basale layer with thin spinosum and granulosum layer and also coated by vary, little and easy detachment corneum layer. While the dermis was included thick granulation tissue which contained the proliferative and migrated cells with a lot of number of big vessels. In addition, the dermis of controlled dogs were hyperemic and surround by mononuclear cells and lymphocytes. The fibrous reticular matrix was arranged irregular collagen bands formation and without hair follicle and sebaceous glands (Figure 8 & 9). Some samples seemed alteration from the hyper-vascularized to hypo-vascularized tissue which converted from immature to mature granulation tissue (Figure 9).
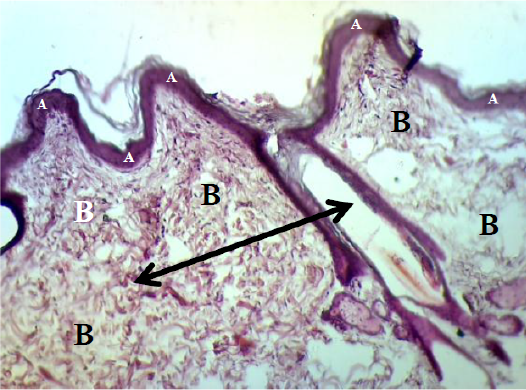
Figure 8: Showed the epidermis (A) consists stratum corneum, stratum granulosum, stratum spinosum and stratum basale. Dermis (B) consists big blood vessel (black arrow). The preparation was by (H&E×40).
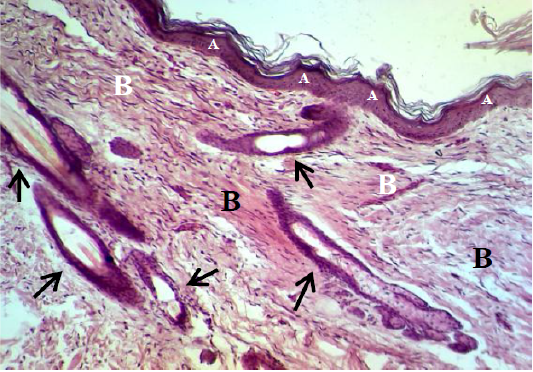
Figure 9: Showed the epidermis (A) consists stratum corneum, stratum granulosum, stratum spinosum and stratum basale. Dermis (B) immature to mature granulation tissue (black arrow). The preparation was by (H&E×40).
Table 3: FGF2 Concentration (Copies/μL)
| Period /day | Control group (M±SE) | Treated group (M±SE) |
| 0 | 107.33±8.07 A | 93.68±6.1 A |
| 7 |
330.46±50.22 B |
376.08±36.42 B |
| 14 | 371.35±30.14 B | 111.95±10.76 A |
| 21 | 112.39±8.78 A | 183.13±8.09 A |
| 28 | 119.52±9.52 A |
254.35±16.54 C |
The capital letters denote significant difference at p<0.01 level.
The percentage of FGF2 proved significant level elevating (p< 0.01) after the first week both groups in however, this difference was not significant in the treated group at the second and third weeks. In contrast, the percentage of FGF2 was returned back to elevate at fourth week in treated group. The differences were significant in control group that continued elevating until the second week, which rebounded at third and fourth weeks.
Discussion
The increasing of TLC in control group occurred due to the destructed tissue debris and organisms invasion to the wound lesion area. The inflammatory processes elongated the inflammatory phase and the inflammatory cells increased for more than 3 weeks. Moreover, some control cases were suffered from high contamination with suppurative wounds. The leukocyte levels of treated animals increased at first week as a result of the destructed tissue debris inflammation alone. Their levels rebounded to the normal state after 14 days. This was because of the propolis activity as anti-microorganisms agent and its contents improved construction and removed the debris for lesion area. All these factors depressed the body wanting for inflammatory cells. These data similar to results of Ammar et al. (2013) that treated the pulmonary airway affected mice by the ethanolic extract of propolis by the 400mg/kg/day orally for 8 days which found that the TLC returned to the normal level. In a similar manner, when Parolia et al. (2010) utilized the raw propolis to cap of the dental pulp. Abbass et al. (2009) and Han et al. (2005) reported that the propolis consist of most elements to enhancement growth tissue and affected treatment. Broughton et al. (2006) stated that the minerals and vitamins are very important for immunity and tissue repair such as synthesize and destruct collagen, epithelialize formation and lymphocyte reaction.
The wound diameter of controlled dogs were reduced at third week. This might be due to the long inflammatory phase which delayed occurrence proliferative phase and generated myofibrobasts which released actin microfilaments and contracted lips of injury. Velnar et al. (2009) demonstrated that the myosin and actin fibers interacting contracted body tissues, at list release connected proteins on the cells edges to pull the extracellular matrix. The reduction in injured wound of treated group observed directly after first week because of the inflammatory stage was short period and occurrence the proliferative stage quickly as well as propolis has an astringent substances, these decreased wound diameter. Kosalec et al. (2003) expressed that the propolis can be used for many medical action such as astringent agents.
The pathological image of control group seemed wound repair with formation of immature granulation tissue because of remains large number of the big vessels with the irregular arrangement of cells and fibers matrix. The repair tissue attempted in some area to convert to mature granulation tissue by reducing of the blood vessels and regulates the arrangement cells and fibers within cellular matrix. Flanagan, (2000) cited that the healthy remodeling injury initiates in about 20 days post injury. At first it has great granulation tissue and redness in color then it matures into little blood vessels and flatted, pale and smooth pattern. Finally, becomes matured scar, it consists of fibrous connective tissue and no vessels, hair follicles or sebaceous glands. The pathological picture of propolis group had complete healing repair tissue. It recognized by the arrangement of skin layers same as to the normal cutaneous pattern but absented appendage structures (hair follicle and sebaceous glands) in the dermis layer that given impression for had been maintaining tissue to rebound to the reorganization and regeneration of skin. These histopathological evaluations similar to observed Han et al. (2005) when used propolis and silver sulfadiazine cream to treat the burned skin in rat.
The FGF2 concentration was multiplied for three times at first and second weeks in the control group, but it became four times at first week only of the propolis group, that played great role in the wound repair by the migration and regeneration of fibroblasts and stimulates neovascularization. Broughton et al. (2006) recorded the FGF releases from lymphocytes, endothelial, mast cells and macrophages, its function is cytoataractic, proliferative of keratinocytes, fibroblasts and revascularization. That growth factor stimulated fibroblasts to create collagen fibers of extracellular matrix and myofibrobasts to produce actin and myosin to contract wound lips. Chan et al. (2000) discovered that occurrence huge amounts formation of collagen type III when injected FGF2 into the tendon patella at dose 100 to 1000 ng. Van De Water et al. (2013) found that myofibroblasts excrete α-actin smooth muscle fiber, that fiber pronounces stressing of tissue with myosin. The FGF2 levels returned to the normal value at third and fourth weeks in control group and at second week in treated group as a result of finished its function in wound repair. It raised gradually at third and fourth weeks in treated dogs about 2 to 3 times more than normal level because of its ability to stimulate fibroblasts to assume the regenerative processes by degradation of irregular matrix fibers and to replace by like arrangement extracellular matrix fibers. Diegelmann and Evans (2004) mentioned that fibroblasts and other cells destruct the collagen molecules via collagenase enzymes into three chains, these chain molecules break down by other proteases. Velnar et al. (2009) revealed that the fibroblasts synthesize the collagen components, they very important in the union and strength of tissues particularly of the proliferation and maturation stages of healing because of the wounded granulation tissue is consisting from 40% collagen type III for the extracellular matrix formation while unwounded skin consisting 80% collagen type I and 25% collagen type III.
Conclusions
The propolis can be used as wound healing agents after preparation. It has minimizing the inflammatory period of the wound healing and hurries assuming proliferating and remodeling stage of the tissue repair. Its repair was enhancement because its ability to rebound to the tissue of semicutaneous naturally as regenerative healing. At last but not list propolis stimulates some somatic cells to release the anabolic cytokines such as basic fibroblast growth factor FGF2. These factors had been the propolis ideal wound healing agent for different animal species and especially dogs.
Acknowledgements
The authors wish to acknowledge the management and staff of University of Kufa, Surgery unit and Theriogenology lab of the Faculty of Veterinary Medicine for their cooperation during this project.
Conflict of interest
Authors declare no conflict of interests.
Authors Contributions
All authors make substantial contributions in various capacities that justify their position as authors in this article. Hussein Ibraheim and Dhurgham Hayder did the field and laboratory work and wrote the manuscript and participated in study conception and design. All authors critically revised the manuscript for important intellectual content and approved the final draft to be submitted.
References






